柱花草SgSTOP1和SgSTOP2基因的克隆与表达分析
English
-
世界范围内,超过50%的可耕作土壤为pH低于5.0的酸性土壤,并主要分布于热带和亚热带地区[1]。在我国,有超过2 000万hm2土壤为酸性土壤,占全国耕地面积的21%[2]。酸性土壤存在着多种限制作物生长的不利因素,主要包括金属离子毒害(如Al3+、Fe3+、Mn2+等)和养分缺乏(如P、Ca、Mg等)[3]。在酸性土壤中,Al3+能通过阻碍细胞分裂和伸长而抑制根系生长,阻碍根系对矿质营养和水分的吸收[4]。在长期进化过程中,植物形成了一系列适应铝毒胁迫的机制。例如,植物受到铝毒胁迫时,可以促进根分泌有机酸,螯合根际铝离子,阻止铝进入细胞。进入细胞的Al3+可被固定在细胞壁内,或被有机酸螯合并隔离在液泡中,从而缓解铝毒害[5]。
锌指转录因子STOP(sensitive to proton rhizotoxicity)是Cys-2-His-2(C2H2)型转录因子家族成员[6]。C2H2锌指蛋白(ZF)是核酸绑定蛋白,定位于细胞核中,起转录调控作用[7]。在拟南芥(Arabidopsis thaliana)中,AtSTOP1含有4个高度保守的C2H2锌指结构域(ZF domain),能调控下游耐铝关键基因表达,如铝激活表达苹果酸转运子AtALMT1(Aluminum activated malate transporter 1)、铝敏感因子AtALS3(Aluminum sensitive 3)和柠檬酸转运蛋白AtMATE1(Multidrug and toxic compound extrusion 1)[8-9]。拟南芥stop1突变体对铝极为敏感,根系生长严重受阻,这可能是由于耐铝相关基因表达受阻所致[8]。近年来,在其他植物中,相继克隆了与AtSTOP1同源的基因,如水稻(Oryza sativa)[10-12]和烟草(Nicotiana tabacum)[13]。因此,STOPs在植物抵御铝毒胁迫中起重要的调控作用。
柱花草(Stylosanthes guianensis)起源于热带和亚热带地区,是重要的豆科牧草,可作为饲草喂养牲畜、作为绿肥覆盖果园和改良土壤等[14-16]。柱花草具有较强的耐铝毒能力,是研究植物适应酸性土壤铝毒胁迫机制的优良材料[17-18]。研究发现,柱花草苹果酸酶基因SgME1(malic enzyme)具有调控根系苹果酸合成螯合铝离子的功能,是柱花草耐酸铝胁迫的重要基因[19]。目前,对柱花草耐铝毒能力评价的研究较多,但其耐铝毒的分子调控机制尚不清楚。因此,本研究以柱花草为材料,克隆获得了柱花草两个锌指转录因子SgSTOP1和SgSTOP2,并对其进行生物信息学和表达模式分析,以期为进一步探索柱花草适应铝毒胁迫的分子调控机制提供基础。
1. 材料与方法
1.1 材料
本研究所用的8份圭亚那柱花草(Stylosanthes guianensis)基因型分别为TPRC2001-1、TF268、TF305、TF387、TF238、TF278、TF207、TF323。柱花草种子由中国热带农业科学院热带作物品种资源研究所热带牧草研究室提供。
1.2 方法
1.2.1 铝处理和相对根长测定
参照Sun等[19]方法,挑选饱满的柱花草种子剥去种壳,80 ℃水浴3 min,冷却后播种于浸润500 μmol·L–1 CaSO4溶液的滤纸上。柱花草幼苗根长至2 cm时,进行2个铝浓度处理,分别为0 μmol·L–1 AlCl3(对照,CK)和60 μmol·L–1 AlCl3(铝处理,Al),每个处理设置4个生物学重复。分别在处理0和24 h后,用根系扫描仪(EPSON,日本)扫描根长图像,并用根系分析软件Image J(National Institutes of Health,美国)统计根长(root length, RL),然后根据公式(RLAl24 h – RLAl0 h)/(RLCK24 h – RLCK0 h) × 100%计算相对根长。
1.2.2 柱花草根系总RNA提取和cDNA合成
参照TRIzol(Invitrogen Inc,美国)方法提取铝处理下TPRC2001-1根系总RNA:取0.1 g根系样品加入1 mL TRIzol提取液,充分研磨后加入0.2 mL氯仿。室温放置5 min后,4 ℃,12 000 r·min–1离心15 min。吸取上清,加入0.5 mL异丙醇,充分混匀后室温放置10 min。4 ℃,12 000 r·min–1离心10 min后,弃上清,用75 %乙醇洗涤沉淀。4 ℃,7 500 r·min–1 离心5 min后,弃上清,风干沉淀,加入DEPC水溶解RNA。
参照M-MLV反转录试剂盒(Invitrogen Inc,美国)方法合成cDNA第一链:在PCR管中加入2 μg RNA、1 μL Oligo(dT)18 (100 nmol·μL–1),并加入ddH2O补足体积至10 μL。于70 ℃保温5 min,冰浴5 min。随后分别加入5 μL M-MLV 5 × Reaction Buffer,1.25 μL dNTPs(10 mmol·L–1),0.5 μL RNase抑制剂(25 U)和1 μL M-MLV RT(200 U)。于42 ℃反应60 min,70 ℃灭活10 min。反应结束后,–20 ℃保存备用。
1.2.3 SgSTOPs全长cDNA克隆
参考柱花草铝毒胁迫转录组结果[18],筛选获得SgSTOP1和SgSTOP2基因全长序列,根据基因序列,设计引物SgSTOP1-TL-F和SgSTOP1-TL-R, SgSTOP2-TL-F和SgSTOP2-TL-R(表1)。以根系cDNA为模板,通过PCR扩增SgSTOP1和SgSTOP2基因。PCR扩增产物经琼脂糖凝胶电泳检测、目的片段回收、连接到克隆载体pEASY-T1(Takara公司)、转化大肠杆菌DH5α和测序分析后,获得SgSTOP1和SgSTOP2全长cDNA序列,并将序列提交至NCBI(https://www.ncbi.nlm.nih.gov/cdd/)。SgSTOP1和SgSTOP2在NCBI上的序列号分别为MH795120和MH795121)。
表 1 SgSTOP1和SgSTOP2基因克隆和定量PCR分析的引物Table 1. Primers for SgSTOP1 and SgSTOP2 cloning and qRT-PCR基因名称 Gene name 引物名称 Primer name 引物序列 Primer sequence (5′-3′) SgSTOP1 SgSTOP1-TL-F ATGGATCCAAAAGTGAGCCTG SgSTOP1-TL-R CTACAGATTATCACAAGTTGATTCACC SgSTOP2 SgSTOP2-TL-F ATGTCCAATCCCAAAACGCAG SgSTOP2-TL-R TTAAATTAAGGGAAACCCTAAAGAATTAAAATC SgSTOP1 SgSTOP1-RT-F ACTACAAGAGGACACACTGCGAC SgSTOP1-RT-R ACGAACAGAGCCATTTATCCTTACCAC SgSTOP2 SgSTOP2-RT-F TCCCTCCATCGCTTCTTATCGG SgSTOP2-RT-R GCGGCTCGATTTTCAGATCCGAC SgEF-1a SgEF-1a-RT-F GTGACCTTCGGACCTTCTGG SgEF-1a-RT-F TGAGGCAACATAACCACGCT 1.2.4 生物信息学分析
运用DNAMAN进行多序列比对分析;利用WoLF PSORT(https://www.genscript.com/wolf-psort.html)进行亚细胞定位预测分析;利用OCTOPUS(http://octopus.cbr.su.se/index.php)预测跨膜结构域和信号肽;采用MAGA(v5.05)进行系统进化树分析;采用MEME在线软件(http://meme-suite.org/)进行蛋白Motif分析;利用NCBI网站进行保守结构域分析。
1.2.5 实时荧光定量PCR分析
运用SYBR Green(Takara,日本)定量试剂盒进行实时荧光定量PCR,用Rotor-Gene 3000系统(Corbett Research,澳大利亚)进行反应。定量PCR反应体系为:10 μL 2 × SYBR Green PCR master mix,6.4 μL Mili-Q水,0.8 μL 10 μmol·L–1的引物,2 μL稀释100倍的cDNA模板。反应程序为:95 ℃ 30 s,94 ℃ 15 s,60 ℃ 15 s,72 ℃ 30 s,40次循环。相对表达量=目的基因表达量/看家基因(SgEF-1a)表达量,目的基因SgSTOP1、SgSTOP2和看家基因SgEF-1a的定量引物如表1所列。
1.3 数据统计分析
采用Microsoft Excel 2003进行作图,用SPSS19.0(SPSS Institute,美国)软件对数据进行统计分析,用平均值±标准误表示测定结果,对铝处理下不同柱花草基因型间进行单因素方差分析,对基因在根和叶、CK和铝处理间分别进行t检验分析。
2. 结果与分析
2.1 铝毒对柱花草相对根长的影响
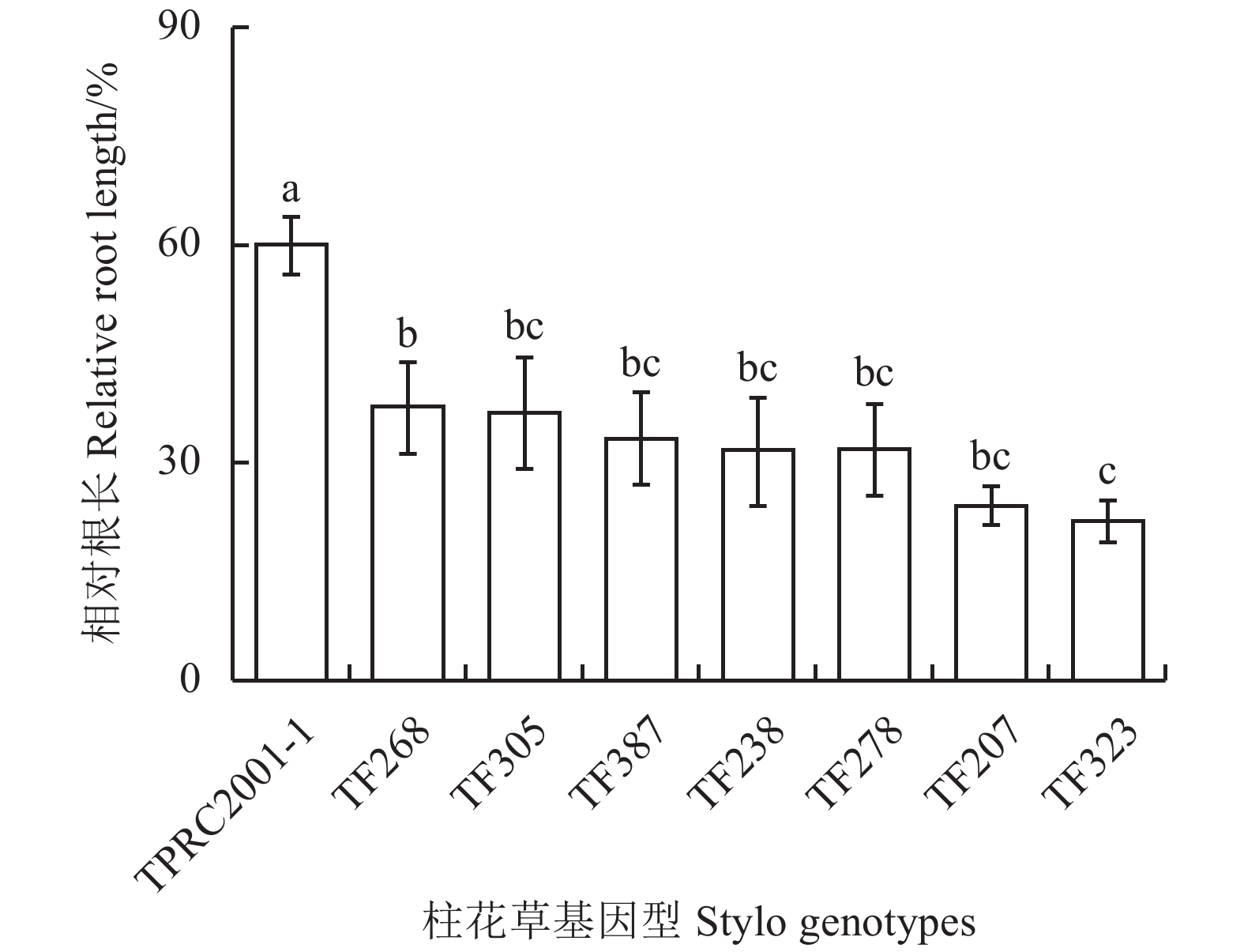
60 μmol·L–1 AlCl3处理显著抑制柱花草根系生长,并且,柱花草的耐铝能力表现出基因型差异。在60 μmol·L–1铝处理下,柱花草TPRC2001-1的相对根长最大,显著大于其他7份基因型(P<0.05);TF268和TF305次之,TF323最小(图1)。这表明,8份试验材料中TPRC2001-1是耐铝柱花草基因型。
2.2 柱花草SgSTOP1和SgSTOP2的克隆与序列分析
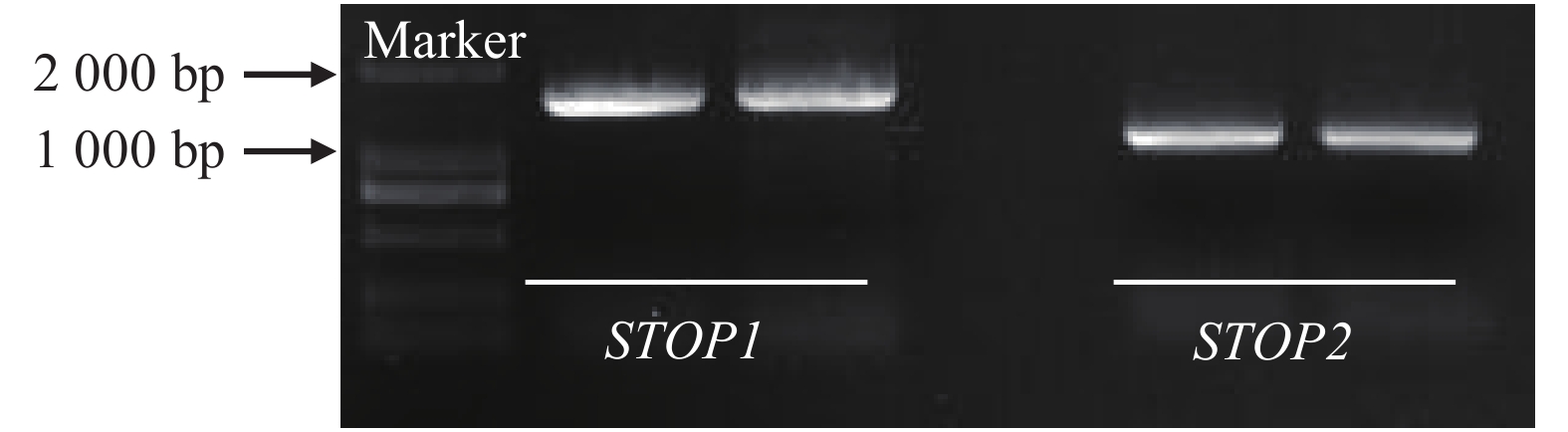
根据转录组测序结果[18],获得SgSTOP1和SgSTOP2基因序列,设计正向和反向引物,以铝毒胁迫下TPRC2001-1根系cDNA为模板,扩增出SgSTOP1和SgSTOP2基因cDNA全长序列。经测序分析发现,SgSTOP1和SgSTOP2基因全长序列与转录组结果一致,基因大小分别为1 515、1 077 bp(图2)。SgSTOP1和SgSTOP2分别编码504、358个氨基酸残基,蛋白分子量分别为56.2和39.7 kDa,理论等电点分别为5.78、5.76,均为亲水性蛋白质。此外,利用在线软件OCTOPUS预测分析发现,SgSTOP1和SgSTOP2均无跨膜结构域和信号肽。利用WoLF PSORT预测分析发现,SgSTOP1和SgSTOP2均定位于细胞核中,表明SgSTOP1和SgSTOP2具备作为转录因子的特点。
2.3 柱花草SgSTOP1和SgSTOP2多序列比对和蛋白保守结构域分析
CDD Tools预测表明,SgSTOP1和SgSTOP2属于ZF-C2H2家族成员,两者分别含有2个Zn绑定位点,2个核酸绑定位点(图3)。将SgSTOP1和SgSTOP2蛋白序列与已报道的不同物种STOPs进行多序列比对分析,发现SgSTOP1和SgSTOP2与其他物种STOPs同源性较高。并且,SgSTOP1和SgSTOP2与其他同源蛋白均具有4个锌指结构域,其中,SgSTOP1的锌指结构均为C2H2型,而SgSTOP2具有3个C2H2型锌指结构域和1个C2HC型锌指结构域。此外,STOPs蛋白在锌指结构域部分较保守,而N端或C端同源性均较低(图4)。
![]() 图 4 SgSTOP1和SgSTOP2与其他STOPs同源蛋白氨基酸比对分析Genebank序列编号分别为柱花草(SgSTOP1, MH795120; SgSTOP2, MH795121)、拟南芥(AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24)、水稻(OsART1, Q2QX40)、烟草(NtSTOP1, S6BF82)、桉树(EguSTOP1, W8VY02)、黑杨(PnSTOP1, S6B8L8)、百脉根(LjSTOP1, S6BUC5)、大豆(GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7)、赤小豆(VuSTOP1, A0A0F7LG52)和高粱(SbSTOP1b, Sb04g023670.1);*表示锌指结构域中的半胱氨酸和组氨酸基。Figure 4. Multiple alignment analysis of SgSTOP1 and SgSTOP2 with homologous plantsGenebank No. Stylosanthes guianensis (SgSTOP1, MH795120; SgSTOP2, MH795121), Arabidopsis thaliana (AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24), Oryza sativa (OsART1, Q2QX40), Nicotiana tabacum (NtSTOP1, S6BF82), Eucalyptus grandis urophylla (EguSTOP1, W8VY02), Populus nigra (PnSTOP1, S6B8L8), Lotus japonicu (LjSTOP1, S6BUC5), Glycine max (GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7), Vigna umbellate (VuSTOP1, A0A0F7LG52), Sorghum bicolor (SbSTOP1b, Sb04g023670.1). * indicate the Cys and His of ZF domain.
图 4 SgSTOP1和SgSTOP2与其他STOPs同源蛋白氨基酸比对分析Genebank序列编号分别为柱花草(SgSTOP1, MH795120; SgSTOP2, MH795121)、拟南芥(AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24)、水稻(OsART1, Q2QX40)、烟草(NtSTOP1, S6BF82)、桉树(EguSTOP1, W8VY02)、黑杨(PnSTOP1, S6B8L8)、百脉根(LjSTOP1, S6BUC5)、大豆(GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7)、赤小豆(VuSTOP1, A0A0F7LG52)和高粱(SbSTOP1b, Sb04g023670.1);*表示锌指结构域中的半胱氨酸和组氨酸基。Figure 4. Multiple alignment analysis of SgSTOP1 and SgSTOP2 with homologous plantsGenebank No. Stylosanthes guianensis (SgSTOP1, MH795120; SgSTOP2, MH795121), Arabidopsis thaliana (AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24), Oryza sativa (OsART1, Q2QX40), Nicotiana tabacum (NtSTOP1, S6BF82), Eucalyptus grandis urophylla (EguSTOP1, W8VY02), Populus nigra (PnSTOP1, S6B8L8), Lotus japonicu (LjSTOP1, S6BUC5), Glycine max (GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7), Vigna umbellate (VuSTOP1, A0A0F7LG52), Sorghum bicolor (SbSTOP1b, Sb04g023670.1). * indicate the Cys and His of ZF domain.2.4 系统进化树分析
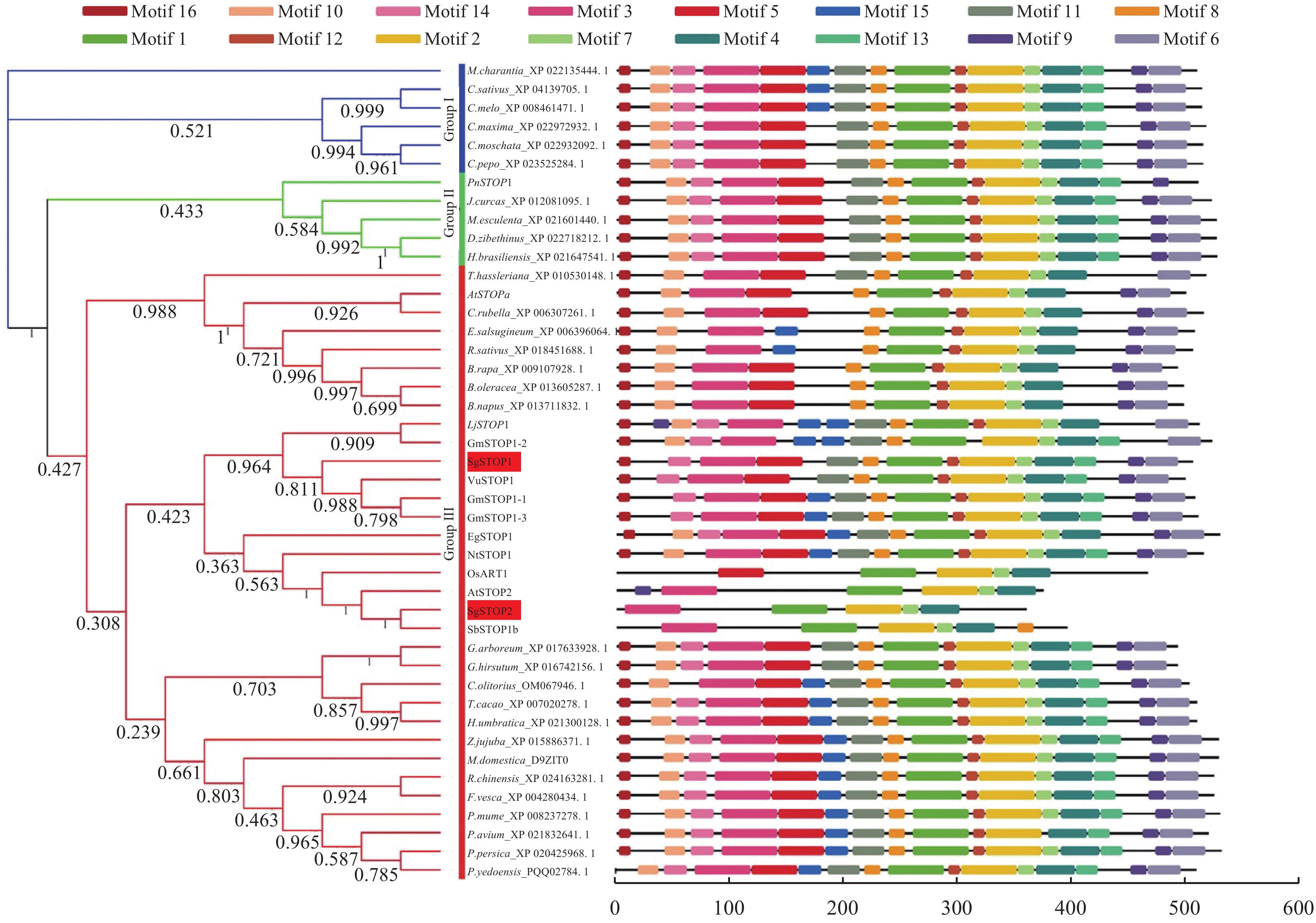
系统进化树分析表明,STOPs蛋白可以被分为Ⅰ、Ⅱ和Ⅲ共3类群(图5)。柱花草SgSTOP1和SgSTOP2同属第Ⅲ类群。其中,SgSTOP1与赤小豆VuSTOP1属同一分枝,相似性最高,达75.9%,而SgSTOP2与水稻ART1、拟南芥AtSTOP2和高粱SgSTOP1b同源性最高。此外,运用MEME软件进行蛋白Motif预测分析,发现STOPs蛋白包含16个保守Motifs(图5)。SgSTOP1和SgSTOP2均含有完整的Motif1(ZF-C2H2结构域),表明两者均属于锌指蛋白家族成员,可能具有转录调控功能。
![]() 图 5 SgSTOP1和SgSTOP2与其他STOPs同源蛋白系统进化树及Motif分析Genebank NO.:苦瓜Momordica charantia(M. charantia_XP 022135444.1),黄瓜Cucumis sativus(C. sativus_XP 004139705.1),香瓜Cucumis melo(C. melo_XP 008461471.1),笋瓜Cucurbita maxima(C. maxima_XP 022972932.1),南瓜Cucurbita moschata(C. moschata_XP 022932092.1),西葫芦Cucurbita pepo(C. pepo_XP 023525284.1),黑杨Populus nigra(PnSTOP1, S6B8L8),麻风树Jatropha curcas(J. curcas_XP 012081095.1),木薯Manihot esculenta (M. esculenta_XP 021601440.1),榴莲Durio zibethinus(D. zibethinus_XP 022718212.1),橡胶Hevea brasiliensis (H. brasiliensis_XP 021647541.1),醉蝶花Tarenaya hassleriana(T. hassleriana_XP 010530148.1),拟南芥Arabidopsis thaliana (AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24),荠菜Capsella rubella (C. rubella_XP 006307261.1),丹参Eutrema salsugineum (E.salsugineum_XP 006396064.1),萝卜Raphanus sativus(R. sativus_XP 018451688.1),芜菁Brassica rapa(B. rapa_XP 009107928.1),百脉根Lotus japonicu(LjSTOP1, S6BUC5),大豆Glycine max(GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7),柱花草 Stylosanthes guianensis (SgSTOP1, MH795120;SgSTOP2, MH795121),饭豆 Vigna umbellate (VuSTOP1, A0A0F7LG52),百桉树 Eucalyptus grandis urophylla (EguSTOP1, W8VY02),烟草 Nicotiana tabacum(NtSTOP1, S6BF82),水稻 Oryza sativa (OsART1, Q2QX40),高粱 Sorghum bicolor(SbSTOP1b, Sb04g023670.1),亚洲棉Gossypium arboretum(G. arboreum_XP 017633928.1),陆地棉Gossypium hirsutum(G. hirsutum_XP 016742156.1),黄麻 Corchorus olitorius(C. olitorius_OMO67946.1),可可 Corchorus olitorius(T. cacao_XP 007020278.1),黑毛黄耆 Herrania umbratical(H. umbratica_XP 021300128.1),灰枣Ziziphus jujube(Z. jujuba_XP 015886371.1),苹果 Malus domestica (M. domestica_D9ZIT0, D9ZIT0),月季Rosa chinensis(R. chinensis_XP 024163281.1),野草莓 Fragaria vesca(F. vesca_XP 004290434.1),梅花Prunus mume(P. mume_XP 008237278.1),甜樱桃 Prunus avium(P. avium_XP 021832641.1),碧桃Prunus persica(P. persica_XP 020425968.1),樱花Prunus yedoensis(P. yedoensis_PQQ02784.1)Figure 5. Phylogenetic tree and Motif analysis of SgSTOP1 and SgSTOP2 with other STOP proteins
图 5 SgSTOP1和SgSTOP2与其他STOPs同源蛋白系统进化树及Motif分析Genebank NO.:苦瓜Momordica charantia(M. charantia_XP 022135444.1),黄瓜Cucumis sativus(C. sativus_XP 004139705.1),香瓜Cucumis melo(C. melo_XP 008461471.1),笋瓜Cucurbita maxima(C. maxima_XP 022972932.1),南瓜Cucurbita moschata(C. moschata_XP 022932092.1),西葫芦Cucurbita pepo(C. pepo_XP 023525284.1),黑杨Populus nigra(PnSTOP1, S6B8L8),麻风树Jatropha curcas(J. curcas_XP 012081095.1),木薯Manihot esculenta (M. esculenta_XP 021601440.1),榴莲Durio zibethinus(D. zibethinus_XP 022718212.1),橡胶Hevea brasiliensis (H. brasiliensis_XP 021647541.1),醉蝶花Tarenaya hassleriana(T. hassleriana_XP 010530148.1),拟南芥Arabidopsis thaliana (AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24),荠菜Capsella rubella (C. rubella_XP 006307261.1),丹参Eutrema salsugineum (E.salsugineum_XP 006396064.1),萝卜Raphanus sativus(R. sativus_XP 018451688.1),芜菁Brassica rapa(B. rapa_XP 009107928.1),百脉根Lotus japonicu(LjSTOP1, S6BUC5),大豆Glycine max(GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7),柱花草 Stylosanthes guianensis (SgSTOP1, MH795120;SgSTOP2, MH795121),饭豆 Vigna umbellate (VuSTOP1, A0A0F7LG52),百桉树 Eucalyptus grandis urophylla (EguSTOP1, W8VY02),烟草 Nicotiana tabacum(NtSTOP1, S6BF82),水稻 Oryza sativa (OsART1, Q2QX40),高粱 Sorghum bicolor(SbSTOP1b, Sb04g023670.1),亚洲棉Gossypium arboretum(G. arboreum_XP 017633928.1),陆地棉Gossypium hirsutum(G. hirsutum_XP 016742156.1),黄麻 Corchorus olitorius(C. olitorius_OMO67946.1),可可 Corchorus olitorius(T. cacao_XP 007020278.1),黑毛黄耆 Herrania umbratical(H. umbratica_XP 021300128.1),灰枣Ziziphus jujube(Z. jujuba_XP 015886371.1),苹果 Malus domestica (M. domestica_D9ZIT0, D9ZIT0),月季Rosa chinensis(R. chinensis_XP 024163281.1),野草莓 Fragaria vesca(F. vesca_XP 004290434.1),梅花Prunus mume(P. mume_XP 008237278.1),甜樱桃 Prunus avium(P. avium_XP 021832641.1),碧桃Prunus persica(P. persica_XP 020425968.1),樱花Prunus yedoensis(P. yedoensis_PQQ02784.1)Figure 5. Phylogenetic tree and Motif analysis of SgSTOP1 and SgSTOP2 with other STOP proteins2.5 SgSTOP1和SgSTOP2基因表达模式分析
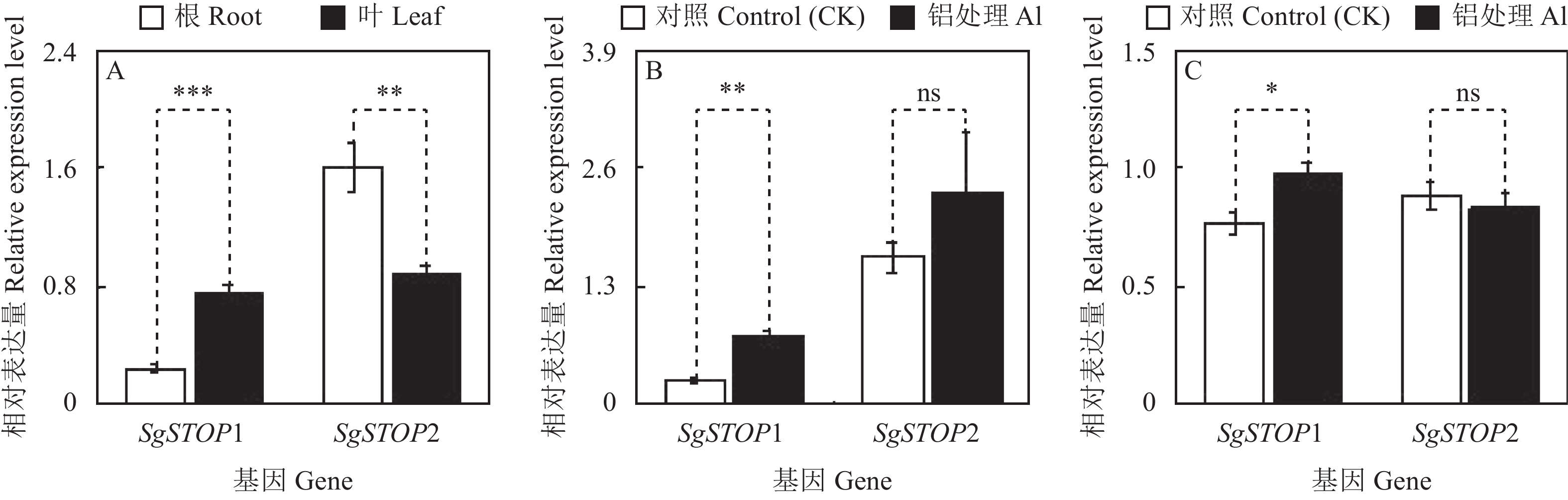
本研究进一步对SgSTOP1和SgSTOP2的表达模式进行分析。结果表明,在正常生长条件下,SgSTOP1和SgSTOP2在根和叶中均有表达,且SgSTOP1在叶部的表达量高于根部,而SgSTOP2在根部的表达量高于叶部(图6A)。相比对照(CK),铝处理(Al)显著增强了SgSTOP1基因在根部和叶部中的表达,但对SgSTOP2基因在根部和叶部的表达均无显著影响(图6B、C)。
![]() 图 6 柱花草SgSTOP1和SgSTOP2基因的表达分析A,基因在CK处理根和叶中的表达量;B,基因在根部中的表达量;C,基因在叶部中的表达量。*,**和***分别表示在0.05、0.01和0.001水平基因表达量在根和叶间(A)、CK和Al处理间(B、C)差异显著。Figure 6. Expression analysis of SgSTOP1 and SgSTOP2 genesA, gene expression levels in leaves and roots of stylo without Al treatment. B and C, gene expression in roots and leaves of stylo with Al treatment, respectively. * indicates significant differences in gene expression between leaves and roots of stylo without Al treatment (A), and roots (B) and leaves (C) of stylo with Al treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
图 6 柱花草SgSTOP1和SgSTOP2基因的表达分析A,基因在CK处理根和叶中的表达量;B,基因在根部中的表达量;C,基因在叶部中的表达量。*,**和***分别表示在0.05、0.01和0.001水平基因表达量在根和叶间(A)、CK和Al处理间(B、C)差异显著。Figure 6. Expression analysis of SgSTOP1 and SgSTOP2 genesA, gene expression levels in leaves and roots of stylo without Al treatment. B and C, gene expression in roots and leaves of stylo with Al treatment, respectively. * indicates significant differences in gene expression between leaves and roots of stylo without Al treatment (A), and roots (B) and leaves (C) of stylo with Al treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001).3. 讨论与结论
铝毒是酸性土壤中限制作物生长的主要因素之一,不同植物或基因型对铝毒害忍耐能力不一样[20]。起源于热带和亚热带地区的植物被认为对酸性土壤铝毒害具有较强的适应性[21]。研究表明,柱花草具有较强的耐铝毒害能力,但表现出基因型差异,其中,柱花草TPRC2001-1耐铝毒能力与水稻“XN1”相当[20]。本研究比较了8份柱花草基因型耐铝能力差异,发现TPRC2001-1的相对根长显著高于其他7份材料,进一步表明TPRC2001-1是耐铝柱花草基因型(图1)。在此基础上,本研究在TPRC2001-1中克隆了柱花草锌指转录因子SgSTOP1和SgSTOP2基因(图2)。
近年来,对C2H2型锌指转录因子STOPs功能研究较为广泛,STOPs基因在植物耐铝毒害中起重要作用[6, 8, 22]。在拟南芥中,具有2个STOPs,在大豆(Glycine max)中,具有3个STOPs,在水稻中,具有1个STOPs的同源蛋白OsART1,在高粱中,具有4个STOPs[22-24]。STOPs转录因子具有高度保守的C2H2锌指结构域,该结构域由23~27个氨基酸残基组成,含有两个保守的半胱氨酸和组氨酸残基[7]。在本研究中,SgSTOP1和SgSTOP2蛋白均属于锌指蛋白家族成员,两者的氨基酸序列均包含4个锌指结构(图3、图4),并且亚细胞定位预测其可能与其他物种STOPs相似,定位于细胞核中。
系统进化树分析表明,柱花草SgSTOP1和SgSTOP2同属第Ⅲ类群,所在类群包括已被证明具有转录调控功能STOPs蛋白,如AtSTOP1、LjSTOP1和NtSTOP1等,它们是耐铝毒胁迫的重要基因[13, 25]。其中,SgSTOP1与调控赤小豆(Vigna umbellata)VuSTOP1相似性最高。研究表明,VuSTOP1具有转录激活功能,能调控柠檬酸转运蛋白VuMATE1的表达,从而增强赤小豆的耐铝性[26]。SgSTOP2与高粱SbSTOP1b同源性最高。Huang等[22]研究发现,SbSTOP1b定位于细胞核中,与SbSTOP1d形成异源二聚体发挥转录活性功能,从而调控SbSTAR2和SbMATE的表达。此外,SgSTOP1和SgSTOP2蛋白序列与其他物种STOPs类似,均包含保守的C2H2锌指结构域Motif1(图5),表明SgSTOP1和SgSTOP2是典型的锌指结构蛋白,可能具有调控下游基因表达的功能。
已有研究表明,铝毒胁迫能够调控植物STOPs基因的表达。如在拟南芥和大豆中,AlCl3处理后,根中AtSTOP1和GmSTOP1的表达量均显著增加[8, 27]。在赤小豆中,Al3+和H+均能诱导VuSTOP1基因的表达[26]。在高粱(Sorghum bicolor)中也发现了类似的结果,AlCl3处理能诱导4个SbSTOP1基因的表达[22]。但是,Yamaji等[10]研究发现,OsART1基因表达不受Al3+影响,但Al3+能增强OsART1下游调控基因OsSTAR1和OsSTAR2的表达,表明OsART1基因在RNA水平上不受Al3+的调控,Al3+可能在蛋白水平上影响OsART1转录活性,进而调控下游基因的表达。在本研究中,铝毒胁迫显著增强SgSTOP1在根和叶中的表达,但铝毒胁迫对SgSTOP2在根和叶中的表达影响不明显(图6),结果暗示了SgSTOP1在转录水平上能响应铝毒胁迫,而SgSTOP2可能与直系同源基因OsART1相似,在转录水平不受Al3+影响,其功能仍需做进一步研究论证。
综上所述,本研究克隆了柱花草SgSTOP1和SgSTOP2基因。亚细胞预测发现,SgSTOP1和SgSTOP2均定位于细胞核中,两者均为ZF-C2H2家族成员,均含有4个锌指结构域。系统进化树分析发现,SgSTOP1与赤小豆VuSTOP1同源性最高,SgSTOP2与高粱SbSTOP1b同源性最高。铝毒处理显著增强SgSTOP1基因在柱花草根和叶中的表达,暗示其参与了柱花草应答铝毒胁迫的过程。为此,后续研究将对SgSTOP1和SgSTOP2的亚细胞定位、基因功能,基因与柱花草耐铝毒能力的相关性进行深入分析,这将有助于进一步揭示柱花草适应铝毒胁迫的分子调控机制。
参考文献
[1] KOCHIAN L V, HOEKENGA O A, PINEROS M A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology, 2004, 55(1): 459-493. doi: 10.1146/annurev.arplant.55.031903.141655
[2] 赵天龙, 解光宁, 张晓霞, 邱林权, 王娜, 张素芝. 酸性土壤上植物应对铝胁迫的过程与机制. 应用生态学报, 2013, 24(10): 3003-3011. ZHAO T L, CHU G N, ZHANG X X, QIU L Q, WANG N, ZHANG S Z. The process and mechanism of plant response to aluminum stress in acid soil. Journal of Applied Ecology, 2013, 24(10): 3003-3011.
[3] ISHITANI M, RAO I, WENZL P, BEEBE S, TOHME J. Integration of genomics approach with traditional breeding towards improving abiotic stress adaptation: drought and aluminum toxicity as case studies. Field Crops Research, 2004, 90(1): 35-45. doi: 10.1016/j.fcr.2004.07.004
[4] FAMOSO A N, CLARK R T, SHAFF J ECRAFT E, MCOUCH S R, KOCHIAN L V. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiology, 2010, 153(4): 1678-1691. doi: 10.1104/pp.110.156794
[5] KOCHIAN L V, PIÑEROS M A, LIU J, MAGALHAES J V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annual Review of Plant Biology, 2015, 66: 571-598. doi: 10.1146/annurev-arplant-043014-114822
[6] IUCHI S, KOYAMA H, IUCHI A, KOBAYASHI Y, KITABAYASHI S, KOBAYASHI Y, IKKA T, HIRAYAMA T, SHINOZAKI K, KOBAYASHI M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(23): 9900. doi: 10.1073/pnas.0700117104
[7] SIEGFRIED B, HEIKO S, ENGLBRECHT C C. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. Bmc Genomics, 2004, 5(1): 1-17. doi: 10.1186/1471-2164-5-1
[8] SAWAKI Y, IUCHI S, KOBAYASHI Y, KOBAYASHI Y, IKKA T, SAKURAI N, FUJITA M, SHINOZAKI K, SHIBATA D, KOBAYASHI M, KOYAMA H. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiology, 2009, 150(1): 281-94. doi: 10.1104/pp.108.134700
[9] LIU J P, MAGALHAES J V, SHAFF JKOCHIAN L V. Aluminium-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant Journal, 2009, 57(3): 389-99. doi: 10.1111/tpj.2009.57.issue-3
[10] YAMAJI N, CHAO F H, NAGAO S, YANO M, SATO Y, NAGAMURA Y, MA J F. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell, 2009, 21(10): 3339-3349. doi: 10.1105/tpc.109.070771
[11] TSUTSUI T, MA J F. Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiology, 2011, 156(2): 925-31. doi: 10.1104/pp.111.175802
[12] CLEMENS S, MA J F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annual Review of Plant Biology, 2016, 67(1): 489. doi: 10.1146/annurev-arplant-043015-112301
[13] OHYAMA Y, ITO H, KOBAYASHI Y, IKKA T, MORITA A, KOBAYASHI M, IMAIZUMI R, AOKI T, KOMATSU K, SAKATA Y, IUCHI S, KOYAMA H. Characterization of AtSTOP1 orthologous genes in tobacco and other plant species. Plant Physiology, 2013, 162(4): 1937-1946. doi: 10.1104/pp.113.218958
[14] SCHULTZE-KRAFT R, RAO I M, PETERS M, CLEMENTS R J, BAI C, LIU G D. Tropical forage legumes for environmental benefits: An overview. Tropical Grasslands-Forrajes Tropicales, 2018, 6(1): 1.
[15] 金莎, 黄世章, 钟毅, 梁梦迪, 陈涛, 王学梅. 香蕉茎叶与柱花草混贮饲料的品质. 草业科学, 2016, 33(3): 512-518. JIN S, HUANG S Z, ZHONG Y, LIANG M D, CHEN T, WANG X M. Quality of banana stem and leaf mixed with Stylosanthes. Pratacultural Science, 2016, 33(3): 512-518.
[16] 蒋亚君, 申晴, 丁西朋, 严琳玲, 刘国道, 白昌军. 柱花草种质资源表型性状的多样性分析. 草业科学, 2017, 34(5): 1032-1041. JIANG Y J, SHEN Q, DING X P, YAN L L, LIU G D, BAI C J. Diversity analysis of phenotypic characters of Stylosanthes germplasm resources. Pratacultural Science, 2017, 34(5): 1032-1041.
[17] NOBLE A D, ORR D M, MIDDLETON C H, ROGERS L G. Legumes in native pasture-asset or liability? A case history with stylo. Tropical Grasslands, 2000, 34: 199-206.
[18] JIANG C, LIU L, LI X, HAN R, WEI Y, YU Y. Insights into aluminum-tolerance pathways in Stylosanthes as revealed by RNA-Seq analysis. Scientific Reports, 2018, 8(1): 6072. doi: 10.1038/s41598-018-24536-3
[19] SUN L L, CUIYUE L, CHEN Z J, LIU P D, TIAN J, LIU G D, LIAO H. Superior aluminum (Al) tolerance of Stylosanthes is achieved mainly by malate synthesis through an Al-enhanced malic enzyme, SgME1. New Phytologist, 2014, 202(1): 209-219. doi: 10.1111/nph.12629
[20] 黄玉婷, 吴亚, 刘大林, 张卫红. 铝胁迫对草本植物生理的影响机制. 草业科学, 2018, 35(6): 1517-1527. ZHANG Y T, WU Y, LIN D H, ZHANG W H. Effects of aluminum stress on the physiology of herbaceous plants. Pratacultural Science, 2018, 35(6): 1517-1527.
[21] METALI F, SALIM K A, BURSLEM D F. Evidence of foliar aluminium accumulation in local, regional and global datasets of wild plants. New Phytologist, 2012, 193(3): 637-649. doi: 10.1111/j.1469-8137.2011.03965.x
[22] HUANG S, GAO J, YOU J. Identification of STOP1-Like proteins associated with aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Frontiers in Plant Science, 2018(9): 258.
[23] FAN W, LOU H Q, YANG J L, ZHENG S J. The roles of STOP1-like transcription factors in aluminum and proton tolerance. Plant Signaling Behavior, 2016, 11(2): e1131371. doi: 10.1080/15592324.2015.1131371
[24] WU W W, LIN Y, CHEN Q Q, PENG W T, PENG J C, TIAN J, LIAO C Y, LIAO H. Functional conservation and divergence of soybean GmSTOP1 members in proton and aluminum tolerance. Frontiers in Plant Science, 2018, 9: 570. doi: 10.3389/fpls.2018.00570
[25] TOKIZAWA M, KOBAYASHI Y, SAITO T, KOBAYASHI M, IUCHI S, NOMOTO M, TADA Y, YAMAMOTO Y Y, KOYAMA H. Sensitive to proton rhizotoxicity1, calmodulin binding transcription activator 2, and other transcription factors are involved in aluminum-activated malate transporter1 expression. Plant Physiology, 2015, 167(3): 991-1003. doi: 10.1104/pp.114.256552
[26] FAN W, LOU H Q, GONG Y L, LIU Y, YANG J L, ZHENG S J. Characterization of an inducible C2H2-type zinc finger transcription factor VuSTOP1 in rice bean (Vigna umbellata) reveals differential regulation between low pH and aluminum tolerance mechanisms. New Phytologist, 2015, 208(2): 456-468. doi: 10.1111/nph.2015.208.issue-2
[27] 丛亚辉, 王婷婷, 柳聚阁, 王宁, 高萌萌, 李艳, 盖钧镒. 大豆耐铝毒候选基因GmSTOP1的克隆与表达分析. 作物学报, 2015, 41(12): 1802-1809. CONG Y H, WANG T T, LIU J G, WANG N, GAO M M, LI Y, GAI J Y. Cloning and expression analysis of the candidate genes GmSTOP1 in soybean for resistance to aluminum toxicity. Crop Journal, 2015, 41(12): 1802-1809.
-
图 4 SgSTOP1和SgSTOP2与其他STOPs同源蛋白氨基酸比对分析
Genebank序列编号分别为柱花草(SgSTOP1, MH795120; SgSTOP2, MH795121)、拟南芥(AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24)、水稻(OsART1, Q2QX40)、烟草(NtSTOP1, S6BF82)、桉树(EguSTOP1, W8VY02)、黑杨(PnSTOP1, S6B8L8)、百脉根(LjSTOP1, S6BUC5)、大豆(GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7)、赤小豆(VuSTOP1, A0A0F7LG52)和高粱(SbSTOP1b, Sb04g023670.1);*表示锌指结构域中的半胱氨酸和组氨酸基。
Figure 4. Multiple alignment analysis of SgSTOP1 and SgSTOP2 with homologous plants
Genebank No. Stylosanthes guianensis (SgSTOP1, MH795120; SgSTOP2, MH795121), Arabidopsis thaliana (AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24), Oryza sativa (OsART1, Q2QX40), Nicotiana tabacum (NtSTOP1, S6BF82), Eucalyptus grandis urophylla (EguSTOP1, W8VY02), Populus nigra (PnSTOP1, S6B8L8), Lotus japonicu (LjSTOP1, S6BUC5), Glycine max (GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7), Vigna umbellate (VuSTOP1, A0A0F7LG52), Sorghum bicolor (SbSTOP1b, Sb04g023670.1). * indicate the Cys and His of ZF domain.
图 5 SgSTOP1和SgSTOP2与其他STOPs同源蛋白系统进化树及Motif分析
Genebank NO.:苦瓜Momordica charantia(M. charantia_XP 022135444.1),黄瓜Cucumis sativus(C. sativus_XP 004139705.1),香瓜Cucumis melo(C. melo_XP 008461471.1),笋瓜Cucurbita maxima(C. maxima_XP 022972932.1),南瓜Cucurbita moschata(C. moschata_XP 022932092.1),西葫芦Cucurbita pepo(C. pepo_XP 023525284.1),黑杨Populus nigra(PnSTOP1, S6B8L8),麻风树Jatropha curcas(J. curcas_XP 012081095.1),木薯Manihot esculenta (M. esculenta_XP 021601440.1),榴莲Durio zibethinus(D. zibethinus_XP 022718212.1),橡胶Hevea brasiliensis (H. brasiliensis_XP 021647541.1),醉蝶花Tarenaya hassleriana(T. hassleriana_XP 010530148.1),拟南芥Arabidopsis thaliana (AtSTOP1, Q9C8N5; AtSTOP2, Q0WT24),荠菜Capsella rubella (C. rubella_XP 006307261.1),丹参Eutrema salsugineum (E.salsugineum_XP 006396064.1),萝卜Raphanus sativus(R. sativus_XP 018451688.1),芜菁Brassica rapa(B. rapa_XP 009107928.1),百脉根Lotus japonicu(LjSTOP1, S6BUC5),大豆Glycine max(GmSTOP1-1, I1LD60; GmSTOP1-2, I1MNY2; GmSTOP1-3, I1NHB7),柱花草 Stylosanthes guianensis (SgSTOP1, MH795120;SgSTOP2, MH795121),饭豆 Vigna umbellate (VuSTOP1, A0A0F7LG52),百桉树 Eucalyptus grandis urophylla (EguSTOP1, W8VY02),烟草 Nicotiana tabacum(NtSTOP1, S6BF82),水稻 Oryza sativa (OsART1, Q2QX40),高粱 Sorghum bicolor(SbSTOP1b, Sb04g023670.1),亚洲棉Gossypium arboretum(G. arboreum_XP 017633928.1),陆地棉Gossypium hirsutum(G. hirsutum_XP 016742156.1),黄麻 Corchorus olitorius(C. olitorius_OMO67946.1),可可 Corchorus olitorius(T. cacao_XP 007020278.1),黑毛黄耆 Herrania umbratical(H. umbratica_XP 021300128.1),灰枣Ziziphus jujube(Z. jujuba_XP 015886371.1),苹果 Malus domestica (M. domestica_D9ZIT0, D9ZIT0),月季Rosa chinensis(R. chinensis_XP 024163281.1),野草莓 Fragaria vesca(F. vesca_XP 004290434.1),梅花Prunus mume(P. mume_XP 008237278.1),甜樱桃 Prunus avium(P. avium_XP 021832641.1),碧桃Prunus persica(P. persica_XP 020425968.1),樱花Prunus yedoensis(P. yedoensis_PQQ02784.1)
Figure 5. Phylogenetic tree and Motif analysis of SgSTOP1 and SgSTOP2 with other STOP proteins
图 6 柱花草SgSTOP1和SgSTOP2基因的表达分析
A,基因在CK处理根和叶中的表达量;B,基因在根部中的表达量;C,基因在叶部中的表达量。*,**和***分别表示在0.05、0.01和0.001水平基因表达量在根和叶间(A)、CK和Al处理间(B、C)差异显著。
Figure 6. Expression analysis of SgSTOP1 and SgSTOP2 genes
A, gene expression levels in leaves and roots of stylo without Al treatment. B and C, gene expression in roots and leaves of stylo with Al treatment, respectively. * indicates significant differences in gene expression between leaves and roots of stylo without Al treatment (A), and roots (B) and leaves (C) of stylo with Al treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
表 1 SgSTOP1和SgSTOP2基因克隆和定量PCR分析的引物
Table 1 Primers for SgSTOP1 and SgSTOP2 cloning and qRT-PCR
基因名称 Gene name 引物名称 Primer name 引物序列 Primer sequence (5′-3′) SgSTOP1 SgSTOP1-TL-F ATGGATCCAAAAGTGAGCCTG SgSTOP1-TL-R CTACAGATTATCACAAGTTGATTCACC SgSTOP2 SgSTOP2-TL-F ATGTCCAATCCCAAAACGCAG SgSTOP2-TL-R TTAAATTAAGGGAAACCCTAAAGAATTAAAATC SgSTOP1 SgSTOP1-RT-F ACTACAAGAGGACACACTGCGAC SgSTOP1-RT-R ACGAACAGAGCCATTTATCCTTACCAC SgSTOP2 SgSTOP2-RT-F TCCCTCCATCGCTTCTTATCGG SgSTOP2-RT-R GCGGCTCGATTTTCAGATCCGAC SgEF-1a SgEF-1a-RT-F GTGACCTTCGGACCTTCTGG SgEF-1a-RT-F TGAGGCAACATAACCACGCT -
[1] KOCHIAN L V, HOEKENGA O A, PINEROS M A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology, 2004, 55(1): 459-493. doi: 10.1146/annurev.arplant.55.031903.141655
[2] 赵天龙, 解光宁, 张晓霞, 邱林权, 王娜, 张素芝. 酸性土壤上植物应对铝胁迫的过程与机制. 应用生态学报, 2013, 24(10): 3003-3011. ZHAO T L, CHU G N, ZHANG X X, QIU L Q, WANG N, ZHANG S Z. The process and mechanism of plant response to aluminum stress in acid soil. Journal of Applied Ecology, 2013, 24(10): 3003-3011.
[3] ISHITANI M, RAO I, WENZL P, BEEBE S, TOHME J. Integration of genomics approach with traditional breeding towards improving abiotic stress adaptation: drought and aluminum toxicity as case studies. Field Crops Research, 2004, 90(1): 35-45. doi: 10.1016/j.fcr.2004.07.004
[4] FAMOSO A N, CLARK R T, SHAFF J ECRAFT E, MCOUCH S R, KOCHIAN L V. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiology, 2010, 153(4): 1678-1691. doi: 10.1104/pp.110.156794
[5] KOCHIAN L V, PIÑEROS M A, LIU J, MAGALHAES J V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annual Review of Plant Biology, 2015, 66: 571-598. doi: 10.1146/annurev-arplant-043014-114822
[6] IUCHI S, KOYAMA H, IUCHI A, KOBAYASHI Y, KITABAYASHI S, KOBAYASHI Y, IKKA T, HIRAYAMA T, SHINOZAKI K, KOBAYASHI M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(23): 9900. doi: 10.1073/pnas.0700117104
[7] SIEGFRIED B, HEIKO S, ENGLBRECHT C C. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. Bmc Genomics, 2004, 5(1): 1-17. doi: 10.1186/1471-2164-5-1
[8] SAWAKI Y, IUCHI S, KOBAYASHI Y, KOBAYASHI Y, IKKA T, SAKURAI N, FUJITA M, SHINOZAKI K, SHIBATA D, KOBAYASHI M, KOYAMA H. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiology, 2009, 150(1): 281-94. doi: 10.1104/pp.108.134700
[9] LIU J P, MAGALHAES J V, SHAFF JKOCHIAN L V. Aluminium-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant Journal, 2009, 57(3): 389-99. doi: 10.1111/tpj.2009.57.issue-3
[10] YAMAJI N, CHAO F H, NAGAO S, YANO M, SATO Y, NAGAMURA Y, MA J F. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell, 2009, 21(10): 3339-3349. doi: 10.1105/tpc.109.070771
[11] TSUTSUI T, MA J F. Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiology, 2011, 156(2): 925-31. doi: 10.1104/pp.111.175802
[12] CLEMENS S, MA J F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annual Review of Plant Biology, 2016, 67(1): 489. doi: 10.1146/annurev-arplant-043015-112301
[13] OHYAMA Y, ITO H, KOBAYASHI Y, IKKA T, MORITA A, KOBAYASHI M, IMAIZUMI R, AOKI T, KOMATSU K, SAKATA Y, IUCHI S, KOYAMA H. Characterization of AtSTOP1 orthologous genes in tobacco and other plant species. Plant Physiology, 2013, 162(4): 1937-1946. doi: 10.1104/pp.113.218958
[14] SCHULTZE-KRAFT R, RAO I M, PETERS M, CLEMENTS R J, BAI C, LIU G D. Tropical forage legumes for environmental benefits: An overview. Tropical Grasslands-Forrajes Tropicales, 2018, 6(1): 1.
[15] 金莎, 黄世章, 钟毅, 梁梦迪, 陈涛, 王学梅. 香蕉茎叶与柱花草混贮饲料的品质. 草业科学, 2016, 33(3): 512-518. JIN S, HUANG S Z, ZHONG Y, LIANG M D, CHEN T, WANG X M. Quality of banana stem and leaf mixed with Stylosanthes. Pratacultural Science, 2016, 33(3): 512-518.
[16] 蒋亚君, 申晴, 丁西朋, 严琳玲, 刘国道, 白昌军. 柱花草种质资源表型性状的多样性分析. 草业科学, 2017, 34(5): 1032-1041. JIANG Y J, SHEN Q, DING X P, YAN L L, LIU G D, BAI C J. Diversity analysis of phenotypic characters of Stylosanthes germplasm resources. Pratacultural Science, 2017, 34(5): 1032-1041.
[17] NOBLE A D, ORR D M, MIDDLETON C H, ROGERS L G. Legumes in native pasture-asset or liability? A case history with stylo. Tropical Grasslands, 2000, 34: 199-206.
[18] JIANG C, LIU L, LI X, HAN R, WEI Y, YU Y. Insights into aluminum-tolerance pathways in Stylosanthes as revealed by RNA-Seq analysis. Scientific Reports, 2018, 8(1): 6072. doi: 10.1038/s41598-018-24536-3
[19] SUN L L, CUIYUE L, CHEN Z J, LIU P D, TIAN J, LIU G D, LIAO H. Superior aluminum (Al) tolerance of Stylosanthes is achieved mainly by malate synthesis through an Al-enhanced malic enzyme, SgME1. New Phytologist, 2014, 202(1): 209-219. doi: 10.1111/nph.12629
[20] 黄玉婷, 吴亚, 刘大林, 张卫红. 铝胁迫对草本植物生理的影响机制. 草业科学, 2018, 35(6): 1517-1527. ZHANG Y T, WU Y, LIN D H, ZHANG W H. Effects of aluminum stress on the physiology of herbaceous plants. Pratacultural Science, 2018, 35(6): 1517-1527.
[21] METALI F, SALIM K A, BURSLEM D F. Evidence of foliar aluminium accumulation in local, regional and global datasets of wild plants. New Phytologist, 2012, 193(3): 637-649. doi: 10.1111/j.1469-8137.2011.03965.x
[22] HUANG S, GAO J, YOU J. Identification of STOP1-Like proteins associated with aluminum tolerance in sweet sorghum (Sorghum bicolor L.). Frontiers in Plant Science, 2018(9): 258.
[23] FAN W, LOU H Q, YANG J L, ZHENG S J. The roles of STOP1-like transcription factors in aluminum and proton tolerance. Plant Signaling Behavior, 2016, 11(2): e1131371. doi: 10.1080/15592324.2015.1131371
[24] WU W W, LIN Y, CHEN Q Q, PENG W T, PENG J C, TIAN J, LIAO C Y, LIAO H. Functional conservation and divergence of soybean GmSTOP1 members in proton and aluminum tolerance. Frontiers in Plant Science, 2018, 9: 570. doi: 10.3389/fpls.2018.00570
[25] TOKIZAWA M, KOBAYASHI Y, SAITO T, KOBAYASHI M, IUCHI S, NOMOTO M, TADA Y, YAMAMOTO Y Y, KOYAMA H. Sensitive to proton rhizotoxicity1, calmodulin binding transcription activator 2, and other transcription factors are involved in aluminum-activated malate transporter1 expression. Plant Physiology, 2015, 167(3): 991-1003. doi: 10.1104/pp.114.256552
[26] FAN W, LOU H Q, GONG Y L, LIU Y, YANG J L, ZHENG S J. Characterization of an inducible C2H2-type zinc finger transcription factor VuSTOP1 in rice bean (Vigna umbellata) reveals differential regulation between low pH and aluminum tolerance mechanisms. New Phytologist, 2015, 208(2): 456-468. doi: 10.1111/nph.2015.208.issue-2
[27] 丛亚辉, 王婷婷, 柳聚阁, 王宁, 高萌萌, 李艳, 盖钧镒. 大豆耐铝毒候选基因GmSTOP1的克隆与表达分析. 作物学报, 2015, 41(12): 1802-1809. CONG Y H, WANG T T, LIU J G, WANG N, GAO M M, LI Y, GAI J Y. Cloning and expression analysis of the candidate genes GmSTOP1 in soybean for resistance to aluminum toxicity. Crop Journal, 2015, 41(12): 1802-1809.
-
期刊类型引用(6)
1. 缪野,刘怀锦,刘莉婷,罗丽娟,陈志坚. 柱花草SgALMT1基因克隆与表达分析. 草地学报. 2024(04): 1078-1086 .  百度学术
百度学术
2. 龙露,汤丹丹,陈玮,谭礼强,陈盛相,唐茜. 茶树STOP基因家族的鉴定及表达模式分析. 茶叶科学. 2024(03): 386-398 .  百度学术
百度学术
3. 王林杰,缪野,邹晓燕,邢玉芬,蒋凌雁,刘国道,陈志坚. 柱花草SgLPR1基因克隆与表达分析. 草业科学. 2023(07): 1856-1865 .  本站查看
本站查看
4. 张永福,徐仕琴,陈姣,杨砚斌,任禛. 葡萄耐铝毒基因STOP1的克隆与表达分析. 西南农业学报. 2022(03): 588-595 .  百度学术
百度学术
5. 段宏利,蒋凌雁,陈志坚,黄春琼. 铝胁迫对狗牙根根系生长和营养元素的影响. 草地学报. 2022(04): 936-942 .  百度学术
百度学术
6. 李季肤,韩佳芮,贾怡丹,张郎织,王文强,王志勇,陈志坚. 地毯草铝响应基因AcABCG1的克隆与表达分析. 草地学报. 2019(05): 1147-1153 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: