镉胁迫下水培杞柳生理特性及镉吸收转运
English
-
在工矿业迅速发展的同时,污水灌溉及工矿业污染导致大量农田土壤镉含量迅速上升[1]。当前,我国耕地污染状况不容乐观,根据2014年发布的全国土壤污染状况调查公报,我国土壤点位镉超标率高达7%[2]。镉是一种生物生长非必需的剧毒重金属元素,极易通过具有较高镉富集系数的作物进入食物链威胁人体健康[3]。自20世纪50年代日本发生因误食“镉米”而导致“水俣病”后,土壤镉污染便引起学者的广泛关注[4-5]。依据我国2018年8月1日实施的最新土壤环境质量标准(GB 15618-2018)[6],全国43%的稻田土壤镉浓度超过其污染风险值[7],由此来看,镉污染土壤修复已迫在眉睫。
植物修复是一种环境友好型的重金属污染土壤修复方式[8-10],而用于土壤污染修复的植物多是重金属的超富集植物与具有较高累积能力的高生物量植物。柳属(Salix)植物是用于镉污染土壤植物修复的一类高生物量植物[11-12]。其中,杞柳(Salix integra)是柳编织品的重要原料,具有适应性强、易繁殖、生长迅速等优点,在我国广泛栽培,具有重要的经济价值。当前有研究表明杞柳对镉胁迫具有一定耐受性且对镉具有一定的吸收能力[13-14]。同时,杞柳可有效促进镉污染土壤中微生物的代谢活性、丰度及多样性指数,还可提高土壤养分水平[15],但杞柳对镉胁迫的生理响应以及对镉的富集转运特性仍有待进一步研究。同时,杞柳作为一种依靠营养体扦插繁殖的植物,其根系建成受镉胁迫的影响仍缺乏更加深入的探索,限制了对杞柳修复镉污染土壤可行性的认识。因此,本研究通过水培扦插杞柳苗的方式,研究镉对杞柳根系构型的影响及其生理响应机制,明确杞柳对镉的吸收转运特性,以期为杞柳在镉污染土壤修复中的应用奠定理论基础。
1. 材料与方法
1.1 试验材料
供试杞柳品种为大青叶,繁育保存于山东农业大学试验站。剪取杞柳的顶端枝条(17 cm),去掉顶部嫩芽(2 cm),扦插于盛有1/2 Hoagland营养液的水培箱中,培养至长出3~5条不定根和嫩叶后,选取长势一致的杞柳苗进行试验。
1.2 试验设计
试验于2018年4月 – 5月在山东农业大学温室中(22 ± 4 ℃)进行。采用水培试验,将长势一致的杞柳苗分别移入1 L的塑料瓶中(每个塑料瓶中移入1棵杞柳苗),加入1/2 Hoagland营养液并将相应浓度的CdCl2 (分析纯)溶解其中,进行镉胁迫试验。以只用1/2 Hoagland营养液的对照处理记为CK,镉处理分别记为C10、C20、C30和C40,对应的镉浓度分别为10、20、30和40 mg·L−1。每个处理4次重复,随机排列。处理14 d后,将植株取出,先将根系浸泡于20 mmol·L−1 EDTA Na溶液中30 min,再用双蒸水洗净以去除螯合附着在根系表面的镉,随后对样品进行检测。
1.3 测定指标与方法
1.3.1 生长指标
分别于处理开始前和处理结束后获取始末生物量(干重),并计算相对生长速率(relative growth rate, RGR)[16]。
$RGR = \frac{{\ln {M_1} - \ln {M_0}}}{d}{\text{。}}$


式中:M0为处理前杞柳干重,M1为处理后杞柳干重,d为处理时间(14 d)。
处理结束后,把植株按照茎、茎表皮、叶片、根系分离,测定根系构型后称取测定生理指标用样品,其余样品置于105 ℃杀青30 min,80 ℃烘干至恒重,得到样品干重。
1.3.2 根系构型
把根系放入根系扫描仪Epson V700的玻璃槽中,注入1 cm深的去离子水使根系充分散开,必要时使用镊子将根系调整散开以避免根系重叠交叉,扫描获取图像,将图像导入Win RHIZO根系分析系统中进行分析获取根系形态数据(根系总长度、平均直径、体积、表面积、分形维数、连接数、节点数、根尖数、分叉数)。
1.3.3 生理指标
参考高俊凤[17]的方法,准确称取0.5 g叶片(根系)置于预冷处理的研钵中,用液氮研磨后,加入5.0 mL磷酸缓冲液(pH 7.0)转移到离心管中,4 ℃离心10 min,收集上清液用于杞柳叶片和根系产生的超氧阴离子活力单位、谷胱甘肽(glutathione, GSH)含量和过氧化氢酶(catalase, CAT)活性的测定;参考Halliwell和Foyer[18]的方法测定谷胱甘肽还原酶(glutathione reductase, GR)活性。
1.3.4 镉的吸收转运
将烘干样品磨碎过0.25 mm筛,分别称取样品(根系0.1 g,叶片、茎表皮和茎分别称取0.2 g)置于石墨坩埚中,在马福炉490 ℃下灰化50 min。将灰化好的材料,加入15 mL混合酸(HCIO4 ꞉ HNO3体积比为1 ꞉ 4),转移至消煮管中消煮至澄清,定容至50 mL容量瓶中,用原子吸收分光光度计测定溶液中镉浓度,计算各器官镉含量,并按以下公式计算镉的去除率[19]、转移系数(translocation factor, TF)[20]、富集系数(bioconcentration factor, BCF)[21]:
去除率 = 植株吸收富集的镉含量/处理前营养液中的镉含量 × 100%;
富集系数 = 植株富集镉浓度/营养液中镉浓度;
转移系数 = 地上部镉浓度/地下部镉浓度。
1.4 数据分析
所有数据均为4次重复的平均值,使用Excel 2010进行做图和数据处理。采用SPSS软件17.0通过Probit回归分析计算IC50 (半抑制浓度),并进行方差分析(多重比较方法为Duncan),比较不同处理间在P < 0.05的显著性水平。
2. 结果与分析
2.1 镉胁迫对杞柳生长及根系构型的影响
2.1.1 镉胁迫对杞柳生长影响
镉胁迫下杞柳的相对生长速率有显著降低(P < 0.05),且抑制作用随胁迫浓度增加而增大(图1),下降幅度为8.88%~59.21%,但C30与C40处理间无显著差异(P > 0.05);分析得出,杞柳镉胁迫的半抑制浓度为25 mg·L−1。在高浓度的镉胁迫下(C30、C40),其生长虽然受到抑制,但仍能存活并保持一定的相对生长率[15.85~17.61 mg·(g·d)−1],表明杞柳对镉胁迫具有较强的耐受性。
![]() 图 1 不同处理杞柳的相对生长率CK为只用1/2 Hoagland营养液的对照处理;C10、C20、C30和C40代表镉浓度分别为10、20、30和40 mg·L−1的镉处理。不同小写字母表示不同镉浓度处理间差异显著(P < 0.05);下同。Figure 1. Relative growth rate of Salix integra following different treatmentsCK indicates the control treatment with 1/2 Hoagland nutrient solution; C10, C20, C30, and C40 indicate cadmium treatment at concentrations of 10, 20, 30, and 40 mg·L−1, respectively. Dfferent lowercase letters indicate a significant difference between different cadmium concentration treatments at the 0.05 level; this is applicable for the following tables and figures as well.
图 1 不同处理杞柳的相对生长率CK为只用1/2 Hoagland营养液的对照处理;C10、C20、C30和C40代表镉浓度分别为10、20、30和40 mg·L−1的镉处理。不同小写字母表示不同镉浓度处理间差异显著(P < 0.05);下同。Figure 1. Relative growth rate of Salix integra following different treatmentsCK indicates the control treatment with 1/2 Hoagland nutrient solution; C10, C20, C30, and C40 indicate cadmium treatment at concentrations of 10, 20, 30, and 40 mg·L−1, respectively. Dfferent lowercase letters indicate a significant difference between different cadmium concentration treatments at the 0.05 level; this is applicable for the following tables and figures as well.杞柳各部位生物量在不同镉浓度胁迫下所受影响不同(表1)。茎和茎表皮生物量在不同镉浓度处理下显著下降(P < 0.05),C10、C20、C30处理间差异不显著(P > 0.05),且均大于C40 (P < 0.05);叶片生物量在C20处理下显著下降(P < 0.05),C10、C20处理显著大于C30、C40 (P < 0.05),C10和C20处理之间、C30和C40处理之间差异均不显著(P > 0.05);杞柳根系生物量与CK相比,仅在C40处理下显著下降43.23%(P < 0.05)。可见,杞柳地上部对镉胁迫的抑制作用更为敏感,而根系对镉胁迫具有更强的耐受性。
表 1 不同处理杞柳各部位生物量Table 1. Biomass of different parts of Salix integra following different treatmentsg 处理
Treatment茎
Stem茎表皮
Epidermis
of stem叶片
Leaf blade根系
RootCK 1.87 ± 0.11a 1.25 ± 0.07a 3.58 ± 0.23a 1.92 ± 0.21ab C10 1.41 ± 0.13b 0.94 ± 0.08b 3.19 ± 0.27ab 2.34 ± 0.17a C20 1.35 ± 0.18b 0.91 ± 0.12b 2.96 ± 0.16b 1.93 ± 0.14ab C30 1.27 ± 0.08b 0.85 ± 0.05b 2.31 ± 0.11c 1.46 ± 0.18bc C40 1.08 ± 0.06c 0.72 ± 0.03c 2.19 ± 0.12c 1.09 ± 0.07c 2.1.2 杞柳根系生长构型
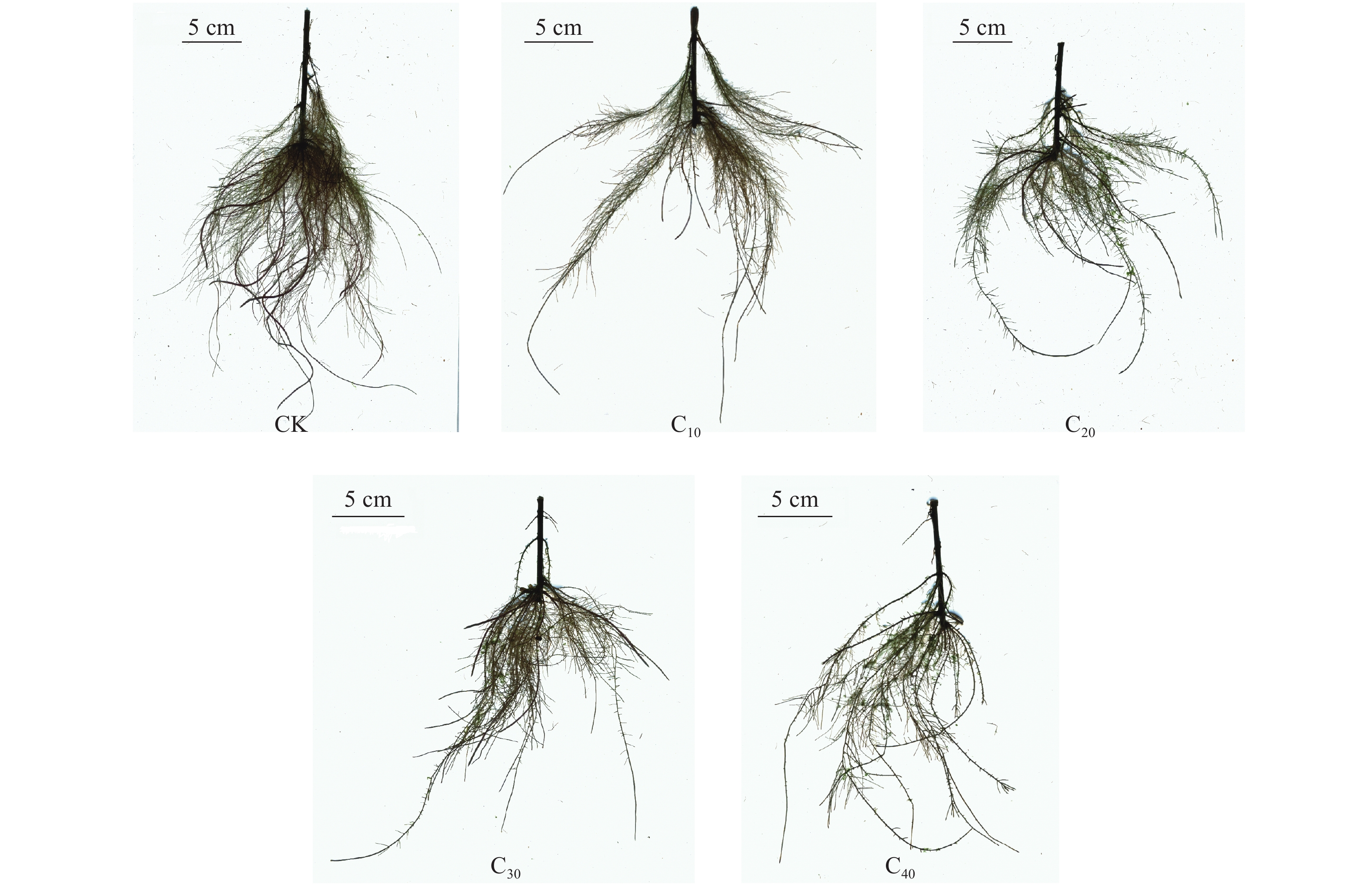
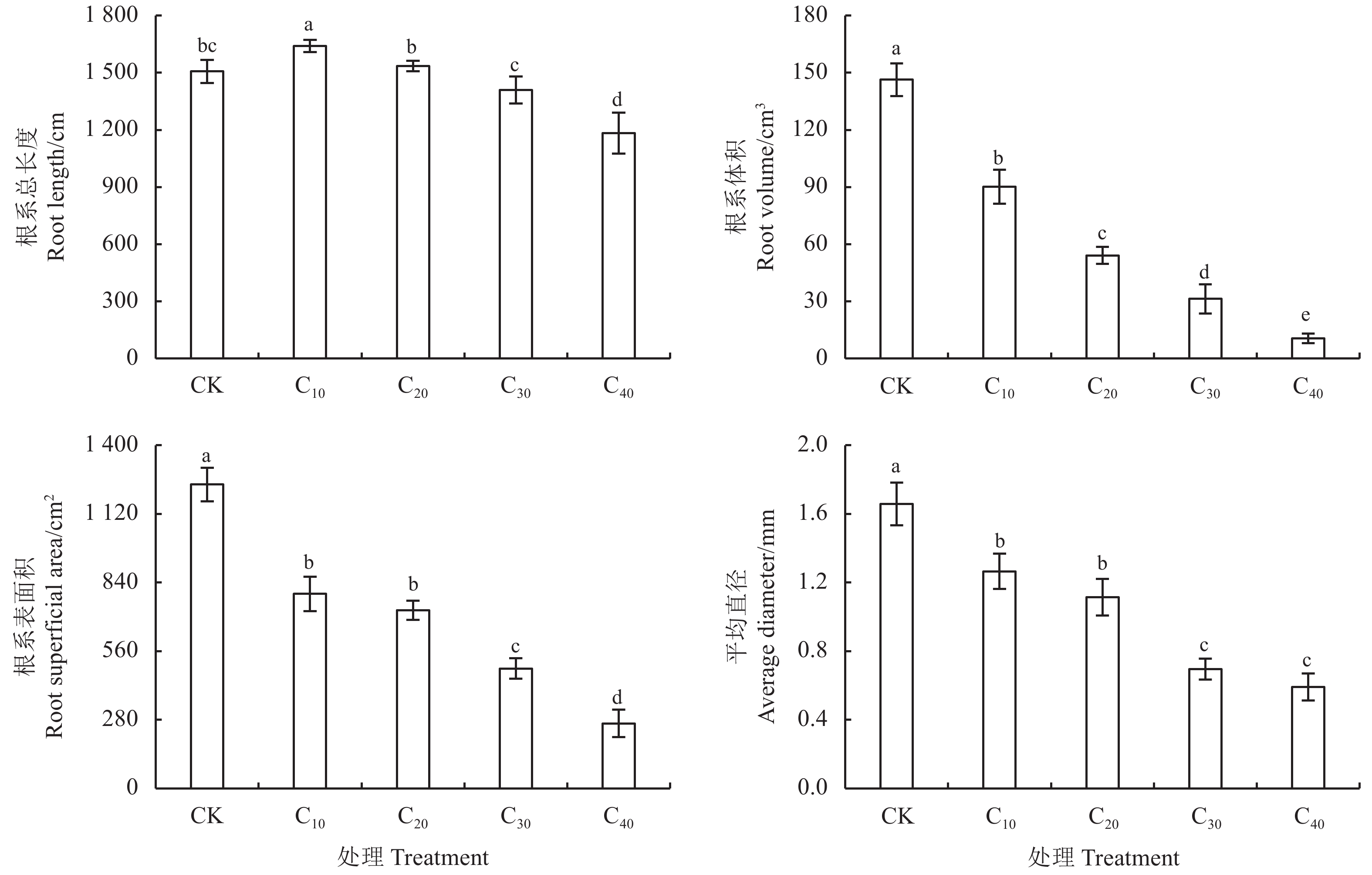
对照处理下杞柳根系发达且侧根茂密,而镉胁迫下侧根随胁迫浓度升高而逐渐稀疏(图2)。镉胁迫对杞柳根系总长度的影响表现为“低促高抑”的趋势,C10较CK显著提高8.85% (P < 0.05);C20、C30与CK差异不显著(P > 0.05),而C40较CK显著下降21.45% (P < 0.05),且镉处理间随浓度提高根系总长度显著下降(P < 0.05) (图3)。较CK,镉胁迫显著降低了根系表面积、平均直径和体积(P < 0.05),且下降幅度随处理浓度提高有增加趋势。C10、C20处理平均直径显著大于C30、C40 (P < 0.05),且C10与C20、C30与C40处理间均无显著差异(P > 0.05);根系体积随镉处理浓度增加而显著降低(P < 0.05);C10、C20处理根系表面积显著大于C30、C40 (P < 0.05),且C10、C20间差异不显著(P > 0.05),但C30处理的根系表面积显著大于C40 (P < 0.05)。因此,杞柳在镉胁迫下根系建成受到抑制,根系表面积、平均直径和体积均下降,尽管根系总长度在低浓度下受刺激增长,但镉胁迫浓度继续增加后,根系总长度也逐渐降低。
较CK处理,镉处理的杞柳分形维数、分叉数均无显著差异(P > 0.05);连接数仅C40处理显著提高22.06% (P < 0.05),其余镉处理均与CK无显著差异(P > 0.05);根系节点数和根尖数在各镉处理下较CK均显著增加(P < 0.05),增加幅度在43.57%~51.98%和17.52%~28.73% (表2)。分形维数在各镉处理间,仅C40较C30显著下降了29.84% (P < 0.05),其余镉胁迫处理间均无显著差异(P > 0.05);杞柳根系连接数、节点数、根尖数和分叉数均在各镉胁迫处理间差异不显著(P > 0.05)。
表 2 不同处理杞柳根系构型指标Table 2. Root configuration index of Salix integra following different treatments处理
Treatment分形维数
Fractal dimension连接数
Linking number节点数
Number of nodes根尖数
Number of root tips分叉数
Number of bifurcationsCK 1.76 ± 0.33ab 9 407 ± 1 781b 5 602 ± 474b 1 650 ± 92b 5 444 ± 379a C10 1.54 ± 0.34ab 10 362 ± 627ab 8 133 ± 694a 2 028 ± 128a 5 527 ± 783a C20 1.47 ± 0.41ab 10 496 ± 398ab 8 514 ± 429a 2 124 ± 117a 5 550 ± 800a C30 1.91 ± 0.41a 10 833 ± 857ab 8 183 ± 220a 2 005 ± 150a 5 584 ± 329a C40 1.34 ± 0.16b 11 482 ± 1 134a 8 043 ± 634a 1 939 ± 158a 5 305 ± 717a 2.2 镉胁迫对杞柳抗氧化酶活性的影响
与CK相比,仅C40处理下杞柳叶片产生超氧阴离子活力单位无显著差异(P > 0.05),其他镉处理下杞柳根系和叶片的产生超氧阴离子活力单位显著上升24.12%~55.12% (P < 0.05),且杞柳根系和叶片中产生超氧阴离子活力单位均随镉处理浓度增加表现为“先增后减”,并在C20处理下达到最高水平(图4)。镉胁迫处理显著提高了杞柳体内的CAT酶活性(P < 0.05),根系和叶片中分别上升了7.60%~26.90%与41.95%~135.29%,但叶片中CAT酶活性随镉处理浓度增加先上升后下降,在C20处理下最高,且C10、C30、C40处理间差异不显著(P > 0.05),而根中CAT酶活性C10、C20处理显著小于C30、C40处理(P < 0.05),但C10与C20、C30与C40处理间差异均不显著(P > 0.05);C20、C30和C40较CK处理显著降低了叶片和根系的GSH含量(P < 0.05),但C10与CK处理间差异不显著(P > 0.05);镉处理显著降低了杞柳根系和叶片GR酶活性35.59%~58.25% (P < 0.05),叶片中GR酶活性C10、C20处理显著大于C30、C40处理(P < 0.05),但C10与C20、C30与C40处理间差异均不显著(P > 0.05),根系GR酶活性在各镉处理间,仅C40显著小于C20处理,下降26.94% (P < 0.05),其余镉处理间均无显著差异(P > 0.05)。
2.3 杞柳对镉的吸收转运
杞柳对镉的吸收转运特性是其对镉污染土壤修复潜力的重要体现,随着镉浓度的不断增加,杞柳地上、地下部镉的富集浓度均持续显著上升(P < 0.05),各个镉胁迫处理下杞柳地上部镉浓度均大于100 mg·kg−1(表3)。在C10处理的去除率最高(27.35%),C20、C30和C40处理的去除率显著小于C10 (P < 0.05),但C20、C30和C40间差异不显著(11.27%~14.97%) (P > 0.05),且杞柳吸收的镉主要富集在叶片中,其次为根系、茎表皮中,茎最低(图5)。C20、C30、C40处理的杞柳转移系数显著大于C10 (P < 0.05),但C20、C30、C40处理间差异不显著(P > 0.05)。此外,杞柳富集系数随镉处理浓度增加而显著下降(P < 0.05),表明高浓度的镉胁迫处理促进镉从根系到地上部的运移,但杞柳的富集能力会受到高浓度镉胁迫的限制。
表 3 不同处理下杞柳植株镉浓度及吸收转运特性Table 3. Cd concentration in plants, absorption and transportation characteristics of Salix integra following different treatments处理
Treatment地下部镉浓度
Underground Cd
concentration/(mg·kg−1)地上部镉浓度
Aboveground Cd
concentration/(mg·kg−1)富集系数
Bioconcentration
factor转移系数
Translocation
factorC10 266.26 ± 27.99d 295.1 ± 21.40d 28.63 ± 2.22a 1.11 ± 0.08b C20 300.11 ± 10.82c 371.2 ± 15.59c 17.67 ± 0.59b 1.24 ± 0.07a C30 409.25 ± 21.79b 494.5 ± 29.61b 15.77 ± 0.71c 1.21 ± 0.11a C40 475.30 ± 13.40a 599.4 ± 19.45a 14.33 ± 0.36d 1.26 ± 0.07a 3. 讨论
杞柳有抗雨涝、生根迅速、根系发达、生长快速、易繁殖等特点,具有作为镉污染修复材料的潜力。已有研究主要通过描述镉胁迫下杞柳生物量的变化以表征杞柳的耐镉性[13-14],而本研究依据不同浓度的镉胁迫处理下杞柳生物量的变化,通过统计分析得出镉胁迫对杞柳的半抑制浓度为25 mg·L−1,进一步明确杞柳对镉胁迫的耐受性。同时,因为杞柳具有一次扦插多年收割的特性,地上部大量富集的镉可通过收割来提取,从而达到修复镉污染的目的[22]。为了进一步了解镉在杞柳植株体内的分配,将杞柳分为茎、茎表皮、叶片、根系四部分进行镉浓度测定,得出镉富集的表现为叶片 > 根系 > 茎表皮 > 茎。而杞柳茎是良好的编制材料,茎中镉的低积累也为其作为产品创造经济效益而无安全风险提供了理论支撑。但是,这与张晓丽等[23]对27种无性柳的研究不一致,其富集的镉多集中于根系,这种差异可能是因品种不同而异。本研究发现,在大于10 mg·L−1的镉污染营养液中,杞柳地上部镉浓度均大于100 mg·kg−1,且其转运系数大于1[24],表明杞柳对镉不仅具有较强的耐受性,而且可作为新型的超积累植物应用于镉污染土壤修复。
镉胁迫下,植物细胞受过量镉的影响产生大量超氧阴离子,从而导致植物体内的D1蛋白受到氧化损伤[25],超氧阴离子生成速率的增加将导致植物衰老死亡[26]。同时,植物体内也会激发相应的抗氧化机制来清除细胞内过量的过氧化物质,过氧化氢酶(CAT)、谷胱甘肽还原酶(GR)和谷胱甘肽(GSH)均为植物抗氧化系统的重要组成部分[27-29]。杞柳在受到镉胁迫后,叶片与根系中的产生超氧阴离子活力单位上升,表明镉胁迫致使杞柳体内形成氧化胁迫。而杞柳叶片和根中CAT酶活性在镉胁迫下上升,有研究表明CAT酶可有效清除植物细胞内的过氧化物质,因此,杞柳通过提高体内CAT酶活性来缓解自身所受氧化胁迫[30]。同时,杞柳叶片CAT酶活性随镉处理浓度增加呈“先升后降”的变化趋势,这与郭艳阳等[31]发现玉米(Zea mays)叶片在氧化胁迫下CAT酶活性随胁迫增强表现为先上升后下降的结果相一致。有研究认为,中度氧化胁迫促使植物体内抗氧化酶活性上升以清除过氧化物质,但严重的氧化胁迫会抑制植物抗氧化酶的活性[32]。GR酶是维持抗坏血酸-谷胱甘肽(AsA-GSH)平衡的关键酶,可将氧化型谷胱甘肽(GS-SG)还原为谷胱甘肽(GSH)来缓解植物所受胁迫[33]。本研究还发现,叶片和根系的GSH含量随着镉浓度增加而逐渐下降,这可能是与其大量合成植物螯合素与镉螯合缓解杞柳镉胁迫密切相关[34]。有研究表明植物和分裂酵母菌可以合成镉结合肽,用于响应和抵御镉胁迫,这种植物螯合素是从GSH衍生而来[35]。此外,杞柳叶片和根系GR活性在镉胁迫下显著降低(P < 0.05),这与王红等[36]发现镉胁迫降低水稻(Oryza sativa) GR酶活性的结果相一致。
杞柳主要通过扦插进行繁育,因此研究镉胁迫对杞柳根系生长发育的影响,可进一步明确其作为镉污染修复植物的潜力[37]。试验表明,杞柳根系的响应分为两方面,一方面是杞柳根系体积、表面积等随镉浓度的上升而受到抑制,根系体积在C10处理时下降38.39% (P < 0.05),并且胁迫的抑制作用随镉浓度增加而增强,在C40处理时下降幅度高达92.73% (P < 0.05),这与王树凤等[14]研究发现的镉胁迫下杞柳根系体积下降的结果相一致;另一方面,杞柳根系分形维数、连接数、节点数、根尖数和分叉数受到镉胁迫的诱导上升,这与李小艳等[38]对杞柳在盐胁迫下根系的响应相一致,在受到盐胁迫后根尖数存在“低促高抑”的变化趋势。因此,杞柳根系在受到镉胁迫后根系体积与表面积等降低,但根系根尖数与节点数等上升,从而在根系生长受阻的情况下维持养分吸收的能力[39]。综上所述,杞柳在镉胁迫下根系生长形态改变以及叶片与根系生理生化反应的调节能力,体现了杞柳对镉胁迫的抗逆响应及其应用于镉污染土壤的修复潜力。
4. 结论
杞柳幼苗对镉胁迫具有较强的耐受性,其镉胁迫的半抑制浓度为25 mg·kg−1。同时,杞柳幼苗具有较高的镉富集转运特性(TF > 1;BCF > 10),吸收的镉主要富集于叶片,有利于通过收割地上部移除污染土壤中的镉。在镉胁迫下,杞柳幼苗叶片和根系的产生超氧阴离子活力单位上升,且CAT酶活性上升以清除过氧化物质,同时叶片和根系GR酶活性与GSH含量下降。此外,虽然镉胁迫抑制了杞柳幼苗的生长,但提高了根系分形维数、连接数、节点数、根尖数和分叉数,促进其根系构型形成,增强根系养分吸收能力保障植物生长。综上所述,杞柳具较强的耐镉性并在镉污染土壤修复中具有较好的应用潜力。
参考文献
[1] HUANG Y, SHENG H, ZHOU P, ZHANG Y Z. Remediation of Cd-contaminated acidic paddy fields with four-year consecutive liming. Ecotoxicology and Environmental Safety, 2020, 188: 109903. doi: 10.1016/j.ecoenv.2019.109903
[2] 刘倩, 卢一富. 无性系金丝柳在北方典型镉污染土壤修复中的应用. 中国农学通报, 2020, 36(34): 90-95. doi: 10.11924/j.issn.1000-6850.casb20191201002 LIU Q, LU Y F. Application of Salix×aureo-pendula in the remediation of typical cadmium contaminated soil in north China. Chinese Agricultural Science Bulletin, 2020, 36(34): 90-95. doi: 10.11924/j.issn.1000-6850.casb20191201002
[3] WANG Y F, SU Y, LU S G. Predicting accumulation of Cd in rice (Oryza sativa L.) and soil threshold concentration of Cd for rice safe production. Science of The Total Environment, 2020, 738: 139805. doi: 10.1016/j.scitotenv.2020.139805
[4] HUANG F, ZHOU H, GU J F, LIU C Y, YANG W J, LIAO B H, ZHOU H. Differences in absorption of cadmium and lead among fourteen sweet potato cultivars and health risk assessment. Ecotoxicology and Environmental Safety, 2020, 203: 111012. doi: 10.1016/j.ecoenv.2020.111012
[5] DOU X K, DAI H P, SKUZA L, WEI S H. Strong accumulation capacity of hyperaccumulator Solanum nigrum L. for low or insoluble Cd compounds in soil and its implication for phytoremediation. Chemosphere, 2020, 260: 127564. doi: 10.1016/j.chemosphere.2020.127564
[6] 生态环境部. 土壤环境质量农用地土壤污染风险管控标准(试行): GB15618-2018. 北京: 中国标准出版社, 2018. Ministry of Ecology and Environment. Soil environmental quality Risk control standard for soil contamination of agricultural land: GB15618-2018. Beijing: Standards Press of China, 2018.
[7] CUI X F, ZHANG Q, TIAN S L, WANG J K, HUANG J H. Status of paddy soil Cd pollution and probabilistic health risk via agricultural contact in China. China Environmental Science, 2021, 41(8): 3878-3886. doi: 10.1016/j.jenvman.2009.01.011
[8] 曾星, 李伟亚, 陈章, 陈国梁, 陈远其, 李志贤. 龙葵修复镉污染土壤的研究进展. 草业科学, 2019, 36(5): 1308-1316. ZENG X, LI W Y, CHEN Z, CHEN G L, CHEN Y Q, LI Z X. Solanum nigrum mediated remediation of Cd contaminated soil. Pratacultural Science, 2019, 36(5): 1308-1316.
[9] 葛依立, 陈心胜, 黄道友, 葛大兵, 邓正苗, 李峰, 谢永宏. 湿地植物水蓼(Polygonum hydropiper L.)对镉的富集特征及生理响应. 生态毒理学报, 2020, 15(2): 190-200. doi: 10.7524/AJE.1673-5897.20190403001 GE Y L, CHEN X S, HUANG D Y, GE D B, DENG Z M, LI F, XIE Y H. Accumulation characteristics and physiological responses of the wetland plant, Polygonum hydropiper L. to cadmium. Asian Journal of Ecotoxicology, 2020, 15(2): 190-200. doi: 10.7524/AJE.1673-5897.20190403001
[10] WEYENS N, SCHELLINGEN K, DUPAE J, CROES S, VAN DER LELIE D, VANGRONSVELD J. Can bacteria associated with willow explain differences in Cd-accumulation capacity between different cultivars? Journal of Biotechnology, 2010, 150(6): 291-292.
[11] CHEN H C, ZHANG S L, WU K J, LI R, HE X R, HE D N, HUANG C, WEI H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. under Cd stress. Ecotoxicology and Environmental Safety, 2020, 187: 109790. doi: 10.1016/j.ecoenv.2019.109790
[12] DOS S U M, WIESHAMMER G, VEGA R, WENZEL W W. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environmental Pollution, 2007, 148(1): 155-165. doi: 10.1016/j.envpol.2006.10.045
[13] 杨卫东, 陈益泰. 不同杞柳品种对镉(Cd)吸收与忍耐的差异. 林业科学研究, 2008, 21(6): 857-861. doi: 10.3321/j.issn:1001-1498.2008.06.022 YANG W D, CHEN Y T. Differences in uptake and tolerance to cadmium in varieties of Salix integra. Forest Research, 2008, 21(6): 857-861. doi: 10.3321/j.issn:1001-1498.2008.06.022
[14] 王树凤, 施翔, 孙海菁, 陈益泰, 杨肖娥. 镉胁迫下杞柳对金属元素的吸收及其根系形态构型特征. 生态学报, 2013, 33(19): 6065-6073. doi: 10.5846/stxb201306091521 WANG S F, SHI X, SUN H J, CHEN Y T, YANG X E. Metal uptake and root morphological changes for two varieties of Salix integra under cadmium stress. Acta Ecologica Sinica, 2013, 33(19): 6065-6073. doi: 10.5846/stxb201306091521
[15] 高卓田, 牛小云, 周健, 王芸, 黄大庄. 种植杞柳在镉污染土壤修复过程中对土壤微生物群落结构和理化性质的影响. 应用与环境生物学报, 2019, 25(3): 501-509. GAO Z T, NIU X Y, ZHOU J, WANG Y, HUANG D Z. Effects of planting Salix integra on the microbial community structure and the physical and chemical properties during cadmium contaminated soil remediation. Chinese Journal of Applied and Environmental Biology, 2019, 25(3): 501-509.
[16] 薛海霞, 李清河, 徐军, 张俊菲. 沙埋对唐古特白刺幼苗生长和生物量分配的影响. 草业科学, 2016, 33(10): 2062-2070. doi: 10.11829/j.issn.1001-0629.2015-0690 XUE H X, LI Q H, XU J, ZHANG J F. Effects of sand burial on growth and biomass allocation of Nitraria tangutorum. Pratacultural Science, 2016, 33(10): 2062-2070. doi: 10.11829/j.issn.1001-0629.2015-0690
[17] 高俊凤. 植物生理学实验指导. 北京: 高等教育出版社, 2006. GAO J F. Plant Physiology Experiment Guide. Beijing: Higher Education Press, 2006.
[18] HALLIWELL B, FOYER C H. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta, 1978, 139(1): 9-17. doi: 10.1007/BF00390803
[19] LOU Y H, LUO H J, HU T, LI H Y, FU J M. Toxic effects, uptake, and translocation of Cd and Pb in perennial ryegrass. Ecotoxicology, 2013, 22(2): 207-214. doi: 10.1007/s10646-012-1017-x
[20] SUN X P, YI H W, CHEN Y P, LOU Y J, PING T, XIE Y H. Effects of different concentrations of Se6+ on selenium absorption, transportation, and distribution of citrus seedlings (C. junos cv. Ziyang xiangcheng). Journal of Plant Nutrition, 2017, 41(2): 168-177.
[21] ANDREAZZA R, BORTOLON L, PIENIZ S, BARCELOS A A, QUADRO M S, CAMARGO F A O. Phytoremediation of vineyard copper-contaminated soil and copper mining waste by a high potential bioenergy crop (Helianthus annus L.). Journal of Plant Nutrition, 2015, 38(10): 1580-1594. doi: 10.1080/01904167.2014.962702
[22] LI J, SUN Y, YIN Y, JI R, WU J C, WANG X R, GUO H Y. Ethyl lactate-EDTA composite system enhances the remediation of the cadmium-contaminated soil by autochthonous willow (Salix × aureo-pendula CL 'J1011') in the lower reaches of the Yangtze River. Journal of Hazardous Materials, 2010, 181(1-3): 673-678. doi: 10.1016/j.jhazmat.2010.05.065
[23] 张晓丽, 翟飞飞, 李伟, 刘俊祥, 钱永强, 韩蕾, 孙振元. 27个柳树无性系对镉的吸收分配特性. 林业科学, 2017, 53(4): 9-17. doi: 10.11707/j.1001-7488.20170402 ZHANG X L, ZHAI F F, LI W, LIU J X, QIAN Y Q, HAN L, SUN Z Y. Characteristics of cadmium absorption and distribution in 27 willow clones. Scientia Silvae Sinicae, 2017, 53(4): 9-17. doi: 10.11707/j.1001-7488.20170402
[24] 韦朝阳, 陈同斌. 重金属超富集植物及植物修复技术研究进展. 生态学报, 2000, 21(7): 1196-1203. WEI C Y, CHEN T B. Hyperaccumulators and phytoremediation of heavy metal contaminated soil: A review of studies in China and abroad. Acta Ecologica Sinica, 2000, 21(7): 1196-1203.
[25] CHEN L, JIA H, TIAN Q, DU L B, GAO Y L, MIAO X X, LIU Y. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynthesis Research, 2012, 112(2): 141-148. doi: 10.1007/s11120-012-9750-9
[26] MCRAE D G, THOMPSON J E. Senescence-dependent changes in superoxide anion production by illuminated chloroplasts from bean leaves. Planta, 1983, 158(3): 185-193. doi: 10.1007/BF01075253
[27] 于德玲, 王昌留. 过氧化氢酶的研究进展. 中国组织化学与细胞化学杂志, 2016, 25(2): 189-194. YU D L, WANG L C. Progress in the research of catalase. Chinese Journal of Histochemistry and Cytochemistry, 2016, 25(2): 189-194.
[28] 林源秀, 顾欣昕, 汤浩茹. 植物谷胱甘肽还原酶的生物学特性及功能. 中国生物化学与分子生物学报, 2013, 29(6): 534-542. LIN Y X, GU X X, TANG H R. Characteristics and biological functions of glutathione reductase in plants. Chinese Journal of Biochemistry and Molecular Biology, 2013, 29(6): 534-542.
[29] SOZONIUK M, NOWAK M, DUDZIAK K, BULAK P, LEŚNIOWSKA-NOWAK J, KOWALCZYK K. Antioxidative system response of pedunculate oak (Quercus robur L.) seedlings to Cd exposure. Physiology and Molecular Biology of Plants, 2019, 25(6): 1377-1384. doi: 10.1007/s12298-019-00712-1
[30] WÓJCIK M, SKÓRZYŃSKAPOLIT E, TUKIENDORF A. Organic acids accumulation and antioxidant enzyme activities in Thlaspi caerulescens under Zn and Cd stress. Plant Growth Regulation, 2006, 48(2): 145-155. doi: 10.1007/s10725-005-5816-4
[31] 郭艳阳, 刘佳, 朱亚利, 柏延文, 李红杰, 薛吉全, 张仁和. 玉米叶片光合和抗氧化酶活性对干旱胁迫的响应. 植物生理学报, 2018, 54(12): 1839-1846. GUO Y Y, LIU J, ZHU Y L, BAI Y W, LI H J, XUE J Q, ZHANG R H. Responses of photosynthetic and antioxidant enzyme activities in maize leaves to drought stress. Plant Physiology Journal, 2018, 54(12): 1839-1846.
[32] BAI L P, SUI F G, GE T D, SUN Z H, LU Y Y, ZHOU G S. Effect of soil drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere, 2006, 16(3): 326-332. doi: 10.1016/S1002-0160(06)60059-3
[33] MÜLLER-SCHÜSSELE S J, WANG R, GÜTLE D D, ROMER J, RODRIGUEZ-FRANCO M, SCHOLZ M, BUCHERT F, LÜTH V M, KOPRIVA S, DÖRMANN P, SCHWARZLÄNDER M, RESKI R, HIPPLER M, MEYER A J. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. The Plant Journal, 2020, 103(3): 1140-1154. doi: 10.1111/tpj.14791
[34] 刘媛, 王妮娅, 张雯, 余佳, 魏虹. 镉胁迫对秋华柳植物螯合肽含量的影响. 浙江大学学报(农业与生命科学版), 2017, 43(3): 298-306. LIU Y, WANG N Y, ZHANG W, YU J, WEI H. Effect of cadmium stress on the content of phytochelatins in Salix variegata. Journal of Zhejiang University (Agriculture and Life Sciences), 2017, 43(3): 298-306.
[35] COBLENZ A, WOLF K. The role of glutathione biosynthesis in heavy metal resistance in the fission yeast Schizosaccharomyces pombe. Fems Microbiology Reviews, 2010, 14(4): 303-308.
[36] 王红, 杨镇, 裴文琪, 李跃, 李学龙, 肇莹, 曹君, 肖军. 功能性微生物制剂对镉胁迫下水稻生长及生理特性的影响. 江苏农业学报, 2016, 32(5): 974-979. doi: 10.3969/j.issn.1000-4440.2016.05.003 WANG H, YANG Z, PEI W Q, LI Y, LI X L, ZHAO Y, CAO J, XIAO J. Growth and physiological characteristics of cadmium-stressed rice influenced by functional microorganism agent. Jiangsu Journal of Agricultural Sciences, 2016, 32(5): 974-979. doi: 10.3969/j.issn.1000-4440.2016.05.003
[37] HE J Y, ZHU C, REN Y F, JIANG D A, SUN Z X. Root morphology and cadmium uptake kinetics of the cadmium-sensitive rice mutant. Biologia Plantarum, 2007, 51(4): 791-794. doi: 10.1007/s10535-007-0162-1
[38] 李小艳, 白瑞琴, 蔺予曼, 贾建宇, 毕良艳. NaCl胁迫对几种柳树生长和生理特性影响的研究. 内蒙古农业大学学报(自然科学版), 2018(5): 7-14. LI X Y, BAI R Q, LIN Y M, JIA J Y, BI L Y. The effects of sodium chloride stress on the growth and physiological characteristics of several willows. Journal of Inner Mongolia Agricultural University (Natural Science Edition), 2018(5): 7-14.
[39] 李乐乐, 李中阳, 吴大付, 班卓昊, 李宝贵, 樊涛, 胡超, 赵志娟, 刘源. 外源物质对镉胁迫下不同品种冬小麦苗期镉吸收特征的影响. 灌溉排水学报, 2021, 40(1): 79-90. LI L L, LI Z Y, WU D F, BAN Z H, LI B G, FAN T, HU C, ZHAO Z J, LIU Y. Cadmium accumulation in wheat of different varieties at seedling stage as impacted by addition of exogenous elements. Journal of Irrigation and Drainage, 2021, 40(1): 79-90.
-
图 1 不同处理杞柳的相对生长率
CK为只用1/2 Hoagland营养液的对照处理;C10、C20、C30和C40代表镉浓度分别为10、20、30和40 mg·L−1的镉处理。不同小写字母表示不同镉浓度处理间差异显著(P < 0.05);下同。
Figure 1. Relative growth rate of Salix integra following different treatments
CK indicates the control treatment with 1/2 Hoagland nutrient solution; C10, C20, C30, and C40 indicate cadmium treatment at concentrations of 10, 20, 30, and 40 mg·L−1, respectively. Dfferent lowercase letters indicate a significant difference between different cadmium concentration treatments at the 0.05 level; this is applicable for the following tables and figures as well.
表 1 不同处理杞柳各部位生物量
Table 1 Biomass of different parts of Salix integra following different treatments
g 处理
Treatment茎
Stem茎表皮
Epidermis
of stem叶片
Leaf blade根系
RootCK 1.87 ± 0.11a 1.25 ± 0.07a 3.58 ± 0.23a 1.92 ± 0.21ab C10 1.41 ± 0.13b 0.94 ± 0.08b 3.19 ± 0.27ab 2.34 ± 0.17a C20 1.35 ± 0.18b 0.91 ± 0.12b 2.96 ± 0.16b 1.93 ± 0.14ab C30 1.27 ± 0.08b 0.85 ± 0.05b 2.31 ± 0.11c 1.46 ± 0.18bc C40 1.08 ± 0.06c 0.72 ± 0.03c 2.19 ± 0.12c 1.09 ± 0.07c 表 2 不同处理杞柳根系构型指标
Table 2 Root configuration index of Salix integra following different treatments
处理
Treatment分形维数
Fractal dimension连接数
Linking number节点数
Number of nodes根尖数
Number of root tips分叉数
Number of bifurcationsCK 1.76 ± 0.33ab 9 407 ± 1 781b 5 602 ± 474b 1 650 ± 92b 5 444 ± 379a C10 1.54 ± 0.34ab 10 362 ± 627ab 8 133 ± 694a 2 028 ± 128a 5 527 ± 783a C20 1.47 ± 0.41ab 10 496 ± 398ab 8 514 ± 429a 2 124 ± 117a 5 550 ± 800a C30 1.91 ± 0.41a 10 833 ± 857ab 8 183 ± 220a 2 005 ± 150a 5 584 ± 329a C40 1.34 ± 0.16b 11 482 ± 1 134a 8 043 ± 634a 1 939 ± 158a 5 305 ± 717a 表 3 不同处理下杞柳植株镉浓度及吸收转运特性
Table 3 Cd concentration in plants, absorption and transportation characteristics of Salix integra following different treatments
处理
Treatment地下部镉浓度
Underground Cd
concentration/(mg·kg−1)地上部镉浓度
Aboveground Cd
concentration/(mg·kg−1)富集系数
Bioconcentration
factor转移系数
Translocation
factorC10 266.26 ± 27.99d 295.1 ± 21.40d 28.63 ± 2.22a 1.11 ± 0.08b C20 300.11 ± 10.82c 371.2 ± 15.59c 17.67 ± 0.59b 1.24 ± 0.07a C30 409.25 ± 21.79b 494.5 ± 29.61b 15.77 ± 0.71c 1.21 ± 0.11a C40 475.30 ± 13.40a 599.4 ± 19.45a 14.33 ± 0.36d 1.26 ± 0.07a -
[1] HUANG Y, SHENG H, ZHOU P, ZHANG Y Z. Remediation of Cd-contaminated acidic paddy fields with four-year consecutive liming. Ecotoxicology and Environmental Safety, 2020, 188: 109903. doi: 10.1016/j.ecoenv.2019.109903
[2] 刘倩, 卢一富. 无性系金丝柳在北方典型镉污染土壤修复中的应用. 中国农学通报, 2020, 36(34): 90-95. doi: 10.11924/j.issn.1000-6850.casb20191201002 LIU Q, LU Y F. Application of Salix×aureo-pendula in the remediation of typical cadmium contaminated soil in north China. Chinese Agricultural Science Bulletin, 2020, 36(34): 90-95. doi: 10.11924/j.issn.1000-6850.casb20191201002
[3] WANG Y F, SU Y, LU S G. Predicting accumulation of Cd in rice (Oryza sativa L.) and soil threshold concentration of Cd for rice safe production. Science of The Total Environment, 2020, 738: 139805. doi: 10.1016/j.scitotenv.2020.139805
[4] HUANG F, ZHOU H, GU J F, LIU C Y, YANG W J, LIAO B H, ZHOU H. Differences in absorption of cadmium and lead among fourteen sweet potato cultivars and health risk assessment. Ecotoxicology and Environmental Safety, 2020, 203: 111012. doi: 10.1016/j.ecoenv.2020.111012
[5] DOU X K, DAI H P, SKUZA L, WEI S H. Strong accumulation capacity of hyperaccumulator Solanum nigrum L. for low or insoluble Cd compounds in soil and its implication for phytoremediation. Chemosphere, 2020, 260: 127564. doi: 10.1016/j.chemosphere.2020.127564
[6] 生态环境部. 土壤环境质量农用地土壤污染风险管控标准(试行): GB15618-2018. 北京: 中国标准出版社, 2018. Ministry of Ecology and Environment. Soil environmental quality Risk control standard for soil contamination of agricultural land: GB15618-2018. Beijing: Standards Press of China, 2018.
[7] CUI X F, ZHANG Q, TIAN S L, WANG J K, HUANG J H. Status of paddy soil Cd pollution and probabilistic health risk via agricultural contact in China. China Environmental Science, 2021, 41(8): 3878-3886. doi: 10.1016/j.jenvman.2009.01.011
[8] 曾星, 李伟亚, 陈章, 陈国梁, 陈远其, 李志贤. 龙葵修复镉污染土壤的研究进展. 草业科学, 2019, 36(5): 1308-1316. ZENG X, LI W Y, CHEN Z, CHEN G L, CHEN Y Q, LI Z X. Solanum nigrum mediated remediation of Cd contaminated soil. Pratacultural Science, 2019, 36(5): 1308-1316.
[9] 葛依立, 陈心胜, 黄道友, 葛大兵, 邓正苗, 李峰, 谢永宏. 湿地植物水蓼(Polygonum hydropiper L.)对镉的富集特征及生理响应. 生态毒理学报, 2020, 15(2): 190-200. doi: 10.7524/AJE.1673-5897.20190403001 GE Y L, CHEN X S, HUANG D Y, GE D B, DENG Z M, LI F, XIE Y H. Accumulation characteristics and physiological responses of the wetland plant, Polygonum hydropiper L. to cadmium. Asian Journal of Ecotoxicology, 2020, 15(2): 190-200. doi: 10.7524/AJE.1673-5897.20190403001
[10] WEYENS N, SCHELLINGEN K, DUPAE J, CROES S, VAN DER LELIE D, VANGRONSVELD J. Can bacteria associated with willow explain differences in Cd-accumulation capacity between different cultivars? Journal of Biotechnology, 2010, 150(6): 291-292.
[11] CHEN H C, ZHANG S L, WU K J, LI R, HE X R, HE D N, HUANG C, WEI H. The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. under Cd stress. Ecotoxicology and Environmental Safety, 2020, 187: 109790. doi: 10.1016/j.ecoenv.2019.109790
[12] DOS S U M, WIESHAMMER G, VEGA R, WENZEL W W. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environmental Pollution, 2007, 148(1): 155-165. doi: 10.1016/j.envpol.2006.10.045
[13] 杨卫东, 陈益泰. 不同杞柳品种对镉(Cd)吸收与忍耐的差异. 林业科学研究, 2008, 21(6): 857-861. doi: 10.3321/j.issn:1001-1498.2008.06.022 YANG W D, CHEN Y T. Differences in uptake and tolerance to cadmium in varieties of Salix integra. Forest Research, 2008, 21(6): 857-861. doi: 10.3321/j.issn:1001-1498.2008.06.022
[14] 王树凤, 施翔, 孙海菁, 陈益泰, 杨肖娥. 镉胁迫下杞柳对金属元素的吸收及其根系形态构型特征. 生态学报, 2013, 33(19): 6065-6073. doi: 10.5846/stxb201306091521 WANG S F, SHI X, SUN H J, CHEN Y T, YANG X E. Metal uptake and root morphological changes for two varieties of Salix integra under cadmium stress. Acta Ecologica Sinica, 2013, 33(19): 6065-6073. doi: 10.5846/stxb201306091521
[15] 高卓田, 牛小云, 周健, 王芸, 黄大庄. 种植杞柳在镉污染土壤修复过程中对土壤微生物群落结构和理化性质的影响. 应用与环境生物学报, 2019, 25(3): 501-509. GAO Z T, NIU X Y, ZHOU J, WANG Y, HUANG D Z. Effects of planting Salix integra on the microbial community structure and the physical and chemical properties during cadmium contaminated soil remediation. Chinese Journal of Applied and Environmental Biology, 2019, 25(3): 501-509.
[16] 薛海霞, 李清河, 徐军, 张俊菲. 沙埋对唐古特白刺幼苗生长和生物量分配的影响. 草业科学, 2016, 33(10): 2062-2070. doi: 10.11829/j.issn.1001-0629.2015-0690 XUE H X, LI Q H, XU J, ZHANG J F. Effects of sand burial on growth and biomass allocation of Nitraria tangutorum. Pratacultural Science, 2016, 33(10): 2062-2070. doi: 10.11829/j.issn.1001-0629.2015-0690
[17] 高俊凤. 植物生理学实验指导. 北京: 高等教育出版社, 2006. GAO J F. Plant Physiology Experiment Guide. Beijing: Higher Education Press, 2006.
[18] HALLIWELL B, FOYER C H. Properties and physiological function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta, 1978, 139(1): 9-17. doi: 10.1007/BF00390803
[19] LOU Y H, LUO H J, HU T, LI H Y, FU J M. Toxic effects, uptake, and translocation of Cd and Pb in perennial ryegrass. Ecotoxicology, 2013, 22(2): 207-214. doi: 10.1007/s10646-012-1017-x
[20] SUN X P, YI H W, CHEN Y P, LOU Y J, PING T, XIE Y H. Effects of different concentrations of Se6+ on selenium absorption, transportation, and distribution of citrus seedlings (C. junos cv. Ziyang xiangcheng). Journal of Plant Nutrition, 2017, 41(2): 168-177.
[21] ANDREAZZA R, BORTOLON L, PIENIZ S, BARCELOS A A, QUADRO M S, CAMARGO F A O. Phytoremediation of vineyard copper-contaminated soil and copper mining waste by a high potential bioenergy crop (Helianthus annus L.). Journal of Plant Nutrition, 2015, 38(10): 1580-1594. doi: 10.1080/01904167.2014.962702
[22] LI J, SUN Y, YIN Y, JI R, WU J C, WANG X R, GUO H Y. Ethyl lactate-EDTA composite system enhances the remediation of the cadmium-contaminated soil by autochthonous willow (Salix × aureo-pendula CL 'J1011') in the lower reaches of the Yangtze River. Journal of Hazardous Materials, 2010, 181(1-3): 673-678. doi: 10.1016/j.jhazmat.2010.05.065
[23] 张晓丽, 翟飞飞, 李伟, 刘俊祥, 钱永强, 韩蕾, 孙振元. 27个柳树无性系对镉的吸收分配特性. 林业科学, 2017, 53(4): 9-17. doi: 10.11707/j.1001-7488.20170402 ZHANG X L, ZHAI F F, LI W, LIU J X, QIAN Y Q, HAN L, SUN Z Y. Characteristics of cadmium absorption and distribution in 27 willow clones. Scientia Silvae Sinicae, 2017, 53(4): 9-17. doi: 10.11707/j.1001-7488.20170402
[24] 韦朝阳, 陈同斌. 重金属超富集植物及植物修复技术研究进展. 生态学报, 2000, 21(7): 1196-1203. WEI C Y, CHEN T B. Hyperaccumulators and phytoremediation of heavy metal contaminated soil: A review of studies in China and abroad. Acta Ecologica Sinica, 2000, 21(7): 1196-1203.
[25] CHEN L, JIA H, TIAN Q, DU L B, GAO Y L, MIAO X X, LIU Y. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynthesis Research, 2012, 112(2): 141-148. doi: 10.1007/s11120-012-9750-9
[26] MCRAE D G, THOMPSON J E. Senescence-dependent changes in superoxide anion production by illuminated chloroplasts from bean leaves. Planta, 1983, 158(3): 185-193. doi: 10.1007/BF01075253
[27] 于德玲, 王昌留. 过氧化氢酶的研究进展. 中国组织化学与细胞化学杂志, 2016, 25(2): 189-194. YU D L, WANG L C. Progress in the research of catalase. Chinese Journal of Histochemistry and Cytochemistry, 2016, 25(2): 189-194.
[28] 林源秀, 顾欣昕, 汤浩茹. 植物谷胱甘肽还原酶的生物学特性及功能. 中国生物化学与分子生物学报, 2013, 29(6): 534-542. LIN Y X, GU X X, TANG H R. Characteristics and biological functions of glutathione reductase in plants. Chinese Journal of Biochemistry and Molecular Biology, 2013, 29(6): 534-542.
[29] SOZONIUK M, NOWAK M, DUDZIAK K, BULAK P, LEŚNIOWSKA-NOWAK J, KOWALCZYK K. Antioxidative system response of pedunculate oak (Quercus robur L.) seedlings to Cd exposure. Physiology and Molecular Biology of Plants, 2019, 25(6): 1377-1384. doi: 10.1007/s12298-019-00712-1
[30] WÓJCIK M, SKÓRZYŃSKAPOLIT E, TUKIENDORF A. Organic acids accumulation and antioxidant enzyme activities in Thlaspi caerulescens under Zn and Cd stress. Plant Growth Regulation, 2006, 48(2): 145-155. doi: 10.1007/s10725-005-5816-4
[31] 郭艳阳, 刘佳, 朱亚利, 柏延文, 李红杰, 薛吉全, 张仁和. 玉米叶片光合和抗氧化酶活性对干旱胁迫的响应. 植物生理学报, 2018, 54(12): 1839-1846. GUO Y Y, LIU J, ZHU Y L, BAI Y W, LI H J, XUE J Q, ZHANG R H. Responses of photosynthetic and antioxidant enzyme activities in maize leaves to drought stress. Plant Physiology Journal, 2018, 54(12): 1839-1846.
[32] BAI L P, SUI F G, GE T D, SUN Z H, LU Y Y, ZHOU G S. Effect of soil drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere, 2006, 16(3): 326-332. doi: 10.1016/S1002-0160(06)60059-3
[33] MÜLLER-SCHÜSSELE S J, WANG R, GÜTLE D D, ROMER J, RODRIGUEZ-FRANCO M, SCHOLZ M, BUCHERT F, LÜTH V M, KOPRIVA S, DÖRMANN P, SCHWARZLÄNDER M, RESKI R, HIPPLER M, MEYER A J. Chloroplasts require glutathione reductase to balance reactive oxygen species and maintain efficient photosynthesis. The Plant Journal, 2020, 103(3): 1140-1154. doi: 10.1111/tpj.14791
[34] 刘媛, 王妮娅, 张雯, 余佳, 魏虹. 镉胁迫对秋华柳植物螯合肽含量的影响. 浙江大学学报(农业与生命科学版), 2017, 43(3): 298-306. LIU Y, WANG N Y, ZHANG W, YU J, WEI H. Effect of cadmium stress on the content of phytochelatins in Salix variegata. Journal of Zhejiang University (Agriculture and Life Sciences), 2017, 43(3): 298-306.
[35] COBLENZ A, WOLF K. The role of glutathione biosynthesis in heavy metal resistance in the fission yeast Schizosaccharomyces pombe. Fems Microbiology Reviews, 2010, 14(4): 303-308.
[36] 王红, 杨镇, 裴文琪, 李跃, 李学龙, 肇莹, 曹君, 肖军. 功能性微生物制剂对镉胁迫下水稻生长及生理特性的影响. 江苏农业学报, 2016, 32(5): 974-979. doi: 10.3969/j.issn.1000-4440.2016.05.003 WANG H, YANG Z, PEI W Q, LI Y, LI X L, ZHAO Y, CAO J, XIAO J. Growth and physiological characteristics of cadmium-stressed rice influenced by functional microorganism agent. Jiangsu Journal of Agricultural Sciences, 2016, 32(5): 974-979. doi: 10.3969/j.issn.1000-4440.2016.05.003
[37] HE J Y, ZHU C, REN Y F, JIANG D A, SUN Z X. Root morphology and cadmium uptake kinetics of the cadmium-sensitive rice mutant. Biologia Plantarum, 2007, 51(4): 791-794. doi: 10.1007/s10535-007-0162-1
[38] 李小艳, 白瑞琴, 蔺予曼, 贾建宇, 毕良艳. NaCl胁迫对几种柳树生长和生理特性影响的研究. 内蒙古农业大学学报(自然科学版), 2018(5): 7-14. LI X Y, BAI R Q, LIN Y M, JIA J Y, BI L Y. The effects of sodium chloride stress on the growth and physiological characteristics of several willows. Journal of Inner Mongolia Agricultural University (Natural Science Edition), 2018(5): 7-14.
[39] 李乐乐, 李中阳, 吴大付, 班卓昊, 李宝贵, 樊涛, 胡超, 赵志娟, 刘源. 外源物质对镉胁迫下不同品种冬小麦苗期镉吸收特征的影响. 灌溉排水学报, 2021, 40(1): 79-90. LI L L, LI Z Y, WU D F, BAN Z H, LI B G, FAN T, HU C, ZHAO Z J, LIU Y. Cadmium accumulation in wheat of different varieties at seedling stage as impacted by addition of exogenous elements. Journal of Irrigation and Drainage, 2021, 40(1): 79-90.
-
期刊类型引用(11)
1. 王慧,李长爱,李亚亮,宁丽丽,刘玲,黄荣. 高浓度镉(Cd)、铅(Pb)胁迫对铜钱草生长和生理生化参数的影响. 生态毒理学报. 2025(01): 440-450 .  百度学术
百度学术
2. 李秀,陈曦,李梦霞,曾心美. 不同木芙蓉品种的镉胁迫响应. 四川林业科技. 2024(01): 58-65 .  百度学术
百度学术
3. 林小兵,周利军,吴多基,尧娟,王斌强,何绍浪,黄尚书,武琳,黄欠如,吴建富. 镉胁迫对粉葛幼苗生长、生理特性及镉富集的影响. 江西农业大学学报. 2024(04): 884-893 .  百度学术
百度学术
4. 何雪,陆引罡,张洁,刘克. 镉胁迫下不同土壤类型对马铃薯生长及镉富集转运特性的影响. 地球与环境. 2023(01): 87-101 .  百度学术
百度学术
5. 马永龙,朱珠,王艳莉,张燕茹,赵安,田青. 中国沙棘幼苗对土壤镉生理耐性和富集转运特征的性别差异. 西北植物学报. 2023(02): 285-294 .  百度学术
百度学术
6. 吴娟子,钱晨,刘智微,钟小仙. 镉胁迫下杂交狼尾草微量元素Fe、Zn、Mn、Cu的吸收和转运. 草业科学. 2023(01): 133-143 .  本站查看
本站查看
7. 王云,赵鹏,李广鑫,胡彩霞,王俊,刘红恩,高巍,秦世玉,睢福庆,李畅. P对小麦Cd和As吸收与转运的影响. 环境科学. 2023(05): 2889-2898 .  百度学术
百度学术
8. 张秀娟,范玟,杨乐,吴楚,杨永胜. 镉胁迫对3种草坪草生理特性及叶片超微结构的影响. 草地学报. 2023(09): 2663-2670 .  百度学术
百度学术
9. 张芬琴,赵连慧,张瑞琪,张潇. 龙葵属两种植物Cd积累特征及其耐性机制对比研究. 河西学院学报. 2023(05): 35-44 .  百度学术
百度学术
10. 李秀,陈曦,李梦霞,陈文凯. 木芙蓉对镉污染土壤的耐性及富集特性. 安徽农学通报. 2023(Z1): 104-108 .  百度学术
百度学术
11. 周倍竹,杨秀玲,王凌晖,唐新瑶,卢立国. 铅胁迫下硅对香樟幼苗生理的调控作用. 热带农业科学. 2022(12): 54-60 .  百度学术
百度学术
其他类型引用(11)




 下载:
下载: