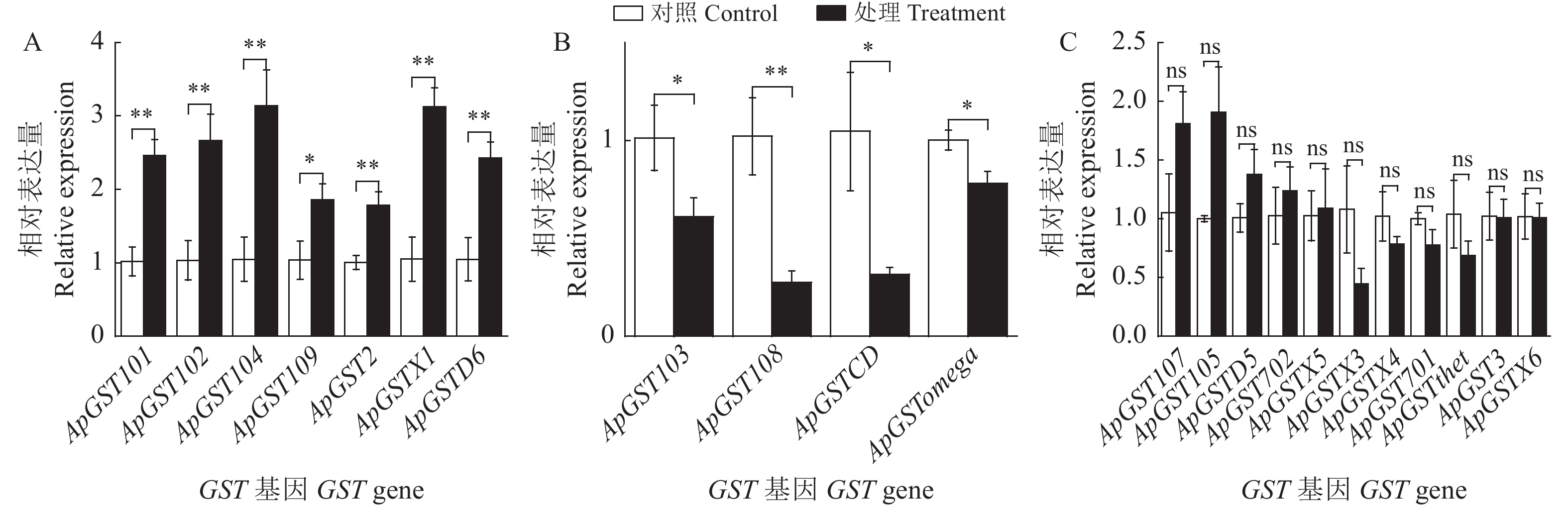
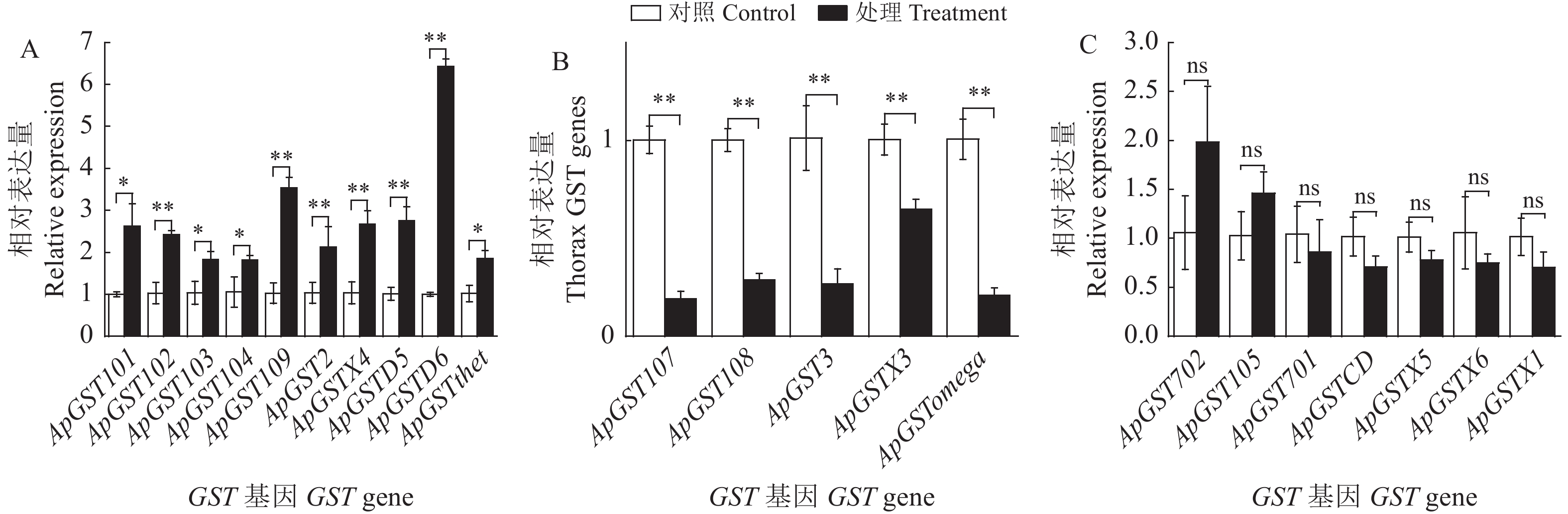
单宁酸对豌豆蚜谷胱甘肽-S-转移酶活性与基因表达的影响
本研究通过测定单宁酸(2 g·L−1)处理豌豆蚜(Acyrthosiphon pisum) 48 h后体内谷胱甘肽-S-转移酶(GST)活性以及不同组织GST基因转录水平的变化,以明确单宁酸对GST活性以及基因表达的影响。结果表明:豌豆蚜取食单宁酸(2 g·L−1) 48 h后,GST活性下降。相比不处理对照,经单宁酸处理后,豌豆蚜头部有7个GST基因上调表达,其中ApGST104、ApGSTX1上调表达3倍左右;胸部有10个GST基因上调表达,其中ApGSTD6上调表达6.4倍;腹部有10个GST基因上调表达,其中ApGST101、ApGST105上调5倍左右;中肠有9个GST基因上调表达,其中ApPGST105上调表达3.2倍。从结果可以看出,单宁酸对豌豆蚜GST基因的表达产生了影响,部分GST基因显著上调表达(P < 0.05),表明GST基因可能在豌豆蚜对单宁酸的代谢中发挥重要的作用。
English
-
藏羊(Ovis aries)原产于我国青藏高原,分布于西藏、青海、甘南藏族自治州、甘孜、阿坝藏族自治州、凉山彝族自治州和云贵高原的部分地区[1]。根据地域差异和自身差异,藏羊可分为山谷型、欧拉型和高原型[2]。藏羊能适应高寒牧区恶劣的生存环境,是青藏高原数量最多的牲畜之一,构成了青藏高原养羊业的主体,也是我国养羊业的重要组成部分[3]。由于高海拔牧区气候恶劣,牧草生长期短、枯草期长,其产量和品质具有明显的季节性差异,无法满足放牧藏羊全年的生长和生产需求,导致藏羊生长发育缓慢,出现“夏肥、秋壮、冬瘦、春死亡”的现象[3-5]。研究表明,通过补饲对藏羊进行营养调控,可以增加藏羊采食量,改善藏羊生长发育,提高藏羊屠宰率、胴体重[4-7],有效达到生产目的。因此,在枯草期对藏羊进行补饲,是改变藏羊越冬困难现状和提高养殖效益的有效手段。
当前藏羊补饲试验中,试验动物发育阶段、补饲饲料类型、补饲水平各不相同,导致研究结果间出现差异,且单个研究结果可能存在试验误差。Meta分析是一种对现有文献进行收集、评价、整理,并综合分析多个试验结果的统计方法,不仅能通过定量的方法汇总多个研究结果,提高结果的可信度,还能解释不同试验结果间的异质性,确保结论的科学性[8]。因此,利用Meta分析,对已有研究结果进行综合统计分析,能够更全面、更客观地评估补饲对藏羊生长及屠宰性能的影响,有效弥补传统文献综述的不足。
1. 材料与方法
系统检索目前已发表的补饲对藏羊生长性能、屠宰性能影响的相关文献,制订文献筛选标准,开展Meta分析。
1.1 文献检索
对PubMed、Web of Science、ScienceDirect、中国知网、万方数据、维普等中英文数据库进行检索,时限为建库至2023年4月12日。采用主题词加自由词结合的检索方式,中文检索词包括藏羊、补饲、生长性能、消化率、采食量、屠宰性能;英文检索词包括Tibetan sheep,supplemental feeding,growth,growth performance,feed intake,digestibility,slaughter performance。
1.2 文献纳入和排除标准
文献纳入标准:1)试验动物必须为藏羊;2)已发表的放牧藏羊随机对照补饲试验,以未补饲组作为对照;3)结果中至少包括藏羊的平均日增重、净增重、干物质采食量、营养物质消化率、屠宰率、胴体重、眼肌面积、胴体脂肪含量值中的一项指标,且研究中给出了试验组与对照组样本量、平均值(Mean)、标准差(SD)或标准误(SE/SEM);4)文献的语言为中文或英文。文献排除标准:1)文献被重复收录、数据相同;2)数据有误或者数据不全的文献。
1.3 数据资料提取
从纳入文献中提取以下数据:1)第一作者、发表年份;2)研究对象、试验动物月龄;3)试验组动物数量、对照组动物数量、试验周期;4)测定指标。
1.4 文献质量评价
采用Cochrane系统评价方法对文献质量进行评价,评价内容包括随机方法、分配隐藏、盲法、数据完整性、选择性报道和其他偏倚来源。使用RevMan5.4.1制作偏倚风险图。
1.5 统计分析
使用Excel 2021对原始数据进行整理归纳,对于没有给出SD的数据,通过Excel换算模板,利用文献中给出的P值、SE/SEM值、样本量进行SD值换算。利用Stata15.1、Graphpad Pism9.5绘制森林图、漏斗图等相关图形。选择标准化均数差(standardized mean difference,SMD)作为效应指标,在95%置信区间(95% confidence interval,95%CI)的设定上,将0值作为无效值,分析结果的95%CI跨越无效值时,使用I 2统计量和卡方检验。当P > 0.10、I 2 < 50%时,表示各研究间有统计学同质性,采用固定效应模型;当P < 0.10、I 2 > 50%时,表示各研究间有统计学异质性,采用随机效应模型。当异质性较大时,采用亚组分析探究异质性来源;采用逐一剔除法进行敏感性分析,评价Meta分析结果的稳健性;根据漏斗图,结合Egger’s检验和Begg’s检验判断是否存在发表偏倚。
2. 结果
2.1 文献筛选流程及质量评估
初检共获得1 689篇文献,经逐层筛选后,最终获得有效文献28篇,其中包括英文文献两篇[9-10]、中文文献26篇[4-7, 11-32] (图1A)。对纳入文献进行质量评估(图1B):在随机分配方法偏倚方面,有2篇文献被定义为高风险,有9篇文献因为没有明确指出分配方法,故将这些文献定义为不明确,其余均为低风险;在实施参与者双盲法偏倚方面,均为低风险;在结果评估盲法偏倚方面,均为低风险;在结果数据不全偏倚与选择性报道偏倚方面,有3篇文献因未以标准形式呈现某些生长性能数据而被定义为高风险,其他均为低风险;在其他偏倚方面,有2篇文献因试验动物数量较少而被定义为高风险。总体来说,纳入本研究的文献质量较高。
2.2 纳入文献的基本特征
本研究旨在综合分析补饲对藏羊生长性能和屠宰性能的影响,由于各研究间补饲饲料类型、补饲时长存在差别,故对纳入文献的补饲饲料种类及试验时间不设限。纳入文献的藏羊共1 728只,其中对照组825只、试验组903只,对照组只放牧不补饲,试验组牧归后对藏羊进行补饲。纳入文献的基本特征如表1所列。
表 1 纳入文献基本特征Table 1. Basic features of the literatures included in the Meta-analysis第一作者
First author发表年份
Year试验动物
Experimental
animal动物月龄
Animal
age/month样本量 Sample size 试验时长
Experimental
period/d测定指标
Measurement对照组
Control group试验组
Test groupSU Yingying [10] 2022 藏公羊
Male Tibetan sheep20 20 60 (1) (4) (5) (6) 郭嵘[29] GUO Rong [29] 2022 藏羊 Tibetan sheep 2 62 65 151 (1) (2) 刘梦等[6]
LIU Meng, et al [6]2022 无角型欧拉羊
Hornless Oula Sheep6 6 6 70 (1) (4) (5) (6) 庄蕾等[13]
ZHUANG Lei, et al [13]2021 无角型欧拉羊
Hornless Oula Sheep6 20 20 70 (1) 周力[15] ZHOU Li [15] 2021 藏羔羊 Tibetan lamb 5 100 100 120 (1) 王彩莲等[23]
WANG Cailian, et al [23]2021 欧拉型藏羊
Oura-type of Tibetan sheep9 10 10 210 (1) 贺钰云[27] HE Yuyun [27] 2019 藏羊 Tibetan sheep 2~3 25 25 68 (1) 王彩莲等[30]
WANG Cailian, et al [30]2018 欧拉型藏羊
Oura-type of Tibetan sheep6 10 10 210 (1) 薛世建[12] XUE Shijian [12] 2018 藏羊 Tibetan sheep 6 20 20 75 (1) JING Xiaoping, et al [9] 2017 藏母羊
Female Tibetan sheep18 6 6 60 (1) 李蕾蕾[28]
LI Leilei [28]2017 欧拉型藏羊
Oura-type of Tibetan sheep18 15 15 70 (1) (2) (3) (4)
(5) (6)光有英[5]
GUANG Youying [5]2017 藏羊
Tibetan sheep20 20 50 (1) (2) 周玉青[14]
ZHOU Yuqing [14]2017 藏羊
Tibetan sheep6 30 30 75 (1) 徐田伟等[4]
XU Tianwei, et al [4]2017 藏羊
Tibetan sheep12 6 6 135 (1) (2) 赵雅丽等[7] ZHAO Yali, et al [7] 2017 藏羊 Tibetan sheep 6 15 15 60 (1) 徐田伟等[19] XU Tianwei, et al [19] 2016 藏羊 Tibetan sheep 36 15 15 70 (1) (2) 卓玉璞等[21]
ZHUO Yupu, et al [21]2016 藏羊
Tibetan sheep12~48 10 10 166 (1) (2) 李生莲等[25]
LI Shenglian, et al [25]2016 藏母羊
Female Tibetan sheep6 30 30 105 (1) (3) (4) (5)
(6)王宏博等[22]
WANG Hongbo, et al [22]2013 藏羔羊
Tibetan lamb6 30 30 100 (1) (2) (3) (4)
(5) (6)李芳芳等[24]
LI Fangfang, et al [24]2012 藏母羊
Female Tibetan sheep175 250 45 (1) (2) 王延基[20]
WANG Yanji [20]2012 藏羊
Tibetan sheep30 30 60 (1) 华着等[11] HUA Zhuo, et al [11] 2012 藏母羊 Female Tibetan sheep 10 10 60 (1) (2) 韩银仓等[18]
HAN Yincang, et al [18]2009 藏公羊
Male Tibetan sheep24 10 10 75 (1) (2) 余忠祥[17]
YU Zhongxiang [17]2009 欧拉型藏羊
Oura-type of Tibetan sheep8 5 5 50 (1) (4) (5) (6) 郑宏[16]
ZHENG Hong [16]2008 藏羊
Tibetan sheep18~24 50 50 30 (1) (2) 李林[26] LI Lin [26] 2007 藏羊 Tibetan sheep 36 10 10 90 (1) (2) 余忠祥等[31]
YU Zhongxiang, et al [31]1998 藏羊
Tibetan sheep18~19 16 16 30 (1) (2) 马玉林等[32] MA Yulin, et al [32] 1996 藏羔羊 Tibetan lamb 6 30 30 (4) 测定指标:(1)平均日增重;(2)净增重;(3)屠宰率;(4)胴体重;(5)眼肌面积;(6)胴体脂肪含量值。
Measurements: (1) average daily gain; (2) net weight gain; (3) dressing percentage; (4) carcass weight; (5) eye muscle area; (6) grass rate value.2.3 补饲对藏羊生长性能的影响
使用Stata软件进行数据分析,I 2统计检验结果显示不同研究间的异质性较大(表2),故选择随机效应模型进行后续Meta分析。结果显示,与未补饲藏羊相比,补饲极显著提高(P < 0.01)藏羊的平均日增重和净增重(图2A、B,表2)。通过亚组分析发现(表3),补饲精料(SMD = 7.23)提高藏羊平均日增重的效果最好,精料 + 粗饲料混合补饲(SMD = 4.79)效果次之,补饲粗饲料(SMD = 4.50)提高藏羊平均日增重的效果与精粗料混合补饲的效果相似(图2C)。
表 2 补饲对藏羊生长性能影响的Meta分析结果Table 2. Meta-analysis of the effects of supplemental feeding on the growth performance of Tibetan sheep测定指标
Measurement研究数量
Numbers of
research/piece标准化均数差 Standard mean difference 异质性检验 Heterogeneity test 效应量
Effect size置信区间(95%CI)
Confidence intervalP I 2/% P 平均日增重 Average daily gain 28 5.48 4.23~6.73 < 0.001 97.6 < 0.1 净增重 Net weight gain 23 5.50 3.88~7.13 < 0.001 98.4 < 0.1 ![]() 图 2 补饲对藏羊生长性能影响的森林图和亚组分析A:平均日增重;B:净增重;C:补饲饲料类型对藏羊平均日增重影响的亚组分析;D:藏羊月龄、补饲精料水平、补饲周期对藏羊平均日增重影响的亚组分析。研究ID表示纳入文献的第一作者及发表年份,标准化均数差(SMD)表示效应量,权重表示对应文献所占权重。图A、B中的SMD = 0的实线,代表无效线;线段表示95% CI,纳入研究的95%CI与无效线相交,说明该研究没有统计学意义;每条线段的中间都有一个黑点代表SMD值,黑点的外围四边形表示权重大小;图形最下方的菱形,表示合并后的效应量。图4同。Figure 2. Forest map and subgroup analysis of the effects of supplemental feeding on the growth performance of Tibetan SheepA: average daily gain; B: net weight gain; C: effects of supplemental feed type on the average daily gain of Tibetan sheep; D: effects of monthly age, concentrate supplementation level, and supplemental feeding days on the average daily gain of Tibetan sheep. The study ID represents the first author and the year of publication of the included literature, SMD represents the magnitude of the effect, and weight represents the weight of the corresponding literature. The solid line of SMD = 0 in Figures A and B represents the invalid line, the line segment represents 95% CI, and the 95% CI of the included study intersects the invalid line, indicating that the study was not statistically significant; a black dot in the middle of each line segment represents the SMD value, and the outer quadrilateral of the black dot indicates the weight. The diamonds at the bottom of the graph represent the combined effect size. This is applicable for Figure 4 as well.表 3 补饲饲料类型对藏羊平均日增重影响的亚组分析结果Table 3. Results of subgroup analysis of the effects of supplemental feed types on the average daily gain of Tibetan sheep
图 2 补饲对藏羊生长性能影响的森林图和亚组分析A:平均日增重;B:净增重;C:补饲饲料类型对藏羊平均日增重影响的亚组分析;D:藏羊月龄、补饲精料水平、补饲周期对藏羊平均日增重影响的亚组分析。研究ID表示纳入文献的第一作者及发表年份,标准化均数差(SMD)表示效应量,权重表示对应文献所占权重。图A、B中的SMD = 0的实线,代表无效线;线段表示95% CI,纳入研究的95%CI与无效线相交,说明该研究没有统计学意义;每条线段的中间都有一个黑点代表SMD值,黑点的外围四边形表示权重大小;图形最下方的菱形,表示合并后的效应量。图4同。Figure 2. Forest map and subgroup analysis of the effects of supplemental feeding on the growth performance of Tibetan SheepA: average daily gain; B: net weight gain; C: effects of supplemental feed type on the average daily gain of Tibetan sheep; D: effects of monthly age, concentrate supplementation level, and supplemental feeding days on the average daily gain of Tibetan sheep. The study ID represents the first author and the year of publication of the included literature, SMD represents the magnitude of the effect, and weight represents the weight of the corresponding literature. The solid line of SMD = 0 in Figures A and B represents the invalid line, the line segment represents 95% CI, and the 95% CI of the included study intersects the invalid line, indicating that the study was not statistically significant; a black dot in the middle of each line segment represents the SMD value, and the outer quadrilateral of the black dot indicates the weight. The diamonds at the bottom of the graph represent the combined effect size. This is applicable for Figure 4 as well.表 3 补饲饲料类型对藏羊平均日增重影响的亚组分析结果Table 3. Results of subgroup analysis of the effects of supplemental feed types on the average daily gain of Tibetan sheep补饲饲料类型
Types of supplementary
feed研究数量
Numbers of
research/
piece标准化均数差 Standard mean difference 异质性检验 Heterogeneity test 效应量
Effect size置信区间(95%CI)
Confidence intervalP I 2/% P 精料 Concentrate 14 7.23 5.02~9.45 < 0.001 97.8 < 0.1 粗饲料 Roughage 4 4.50 2.48~6.53 < 0.001 94.4 < 0.1 精料 + 粗饲料 Concentrate + roughage 2 4.79 3.53~6.05 < 0.001 87.1 0.192 营养舔砖 Nutritional brick 5 3.66 1.36~5.95 0.02 96.3 < 0.1 尿素补料 Urea 3 1.84 0.85~2.82 < 0.001 59.6 0.084 鉴于精料补饲的效果最好,故以藏羊月龄、精料补饲水平和补饲周期作为分组依据进行亚组分析(表4)。根据纳入文献中的信息,将藏羊月龄划分为 < 6月龄、6月龄、9月龄及18~36月龄。结果表明,精料补饲提高 < 6月龄藏羊平均日增重(SMD = 15.94)的效果最好,提高18~36月龄藏羊平均日增重(SMD = 3.88)的效果最差,提高6月龄(SMD = 4.71)和9月龄(SMD = 4.70)藏羊平均日增重的效果相似。此外,精料补饲提高藏羊平均日增重的效果随着精料补饲水平及补饲周期的增加而提高,补饲效果表现为0.2~0.4 kg·d−1 > 0.15 kg·d−1 > 0.1 kg·d−1;180~210 d > 60~90 d (图2D)。
表 4 补饲精料对藏羊平均日增重影响的亚组分析结果Table 4. Results of subgroup analysis of the effects of supplementing concentrate on the average daily gain in Tibetan sheep分组依据
Group研究数量
Numbers of
research/
piece标准化均数差 Standard mean difference 异质性检验 Heterogeneity test 效应量
Effect size置信区间(95%CI)
Confidence intervalP I 2/% P 藏羊月龄
Tibetan sheep
age/month< 6 3 15.94 8.50~23.39 < 0.001 98.9 < 0.1 6 4 4.71 2.74~6.67 < 0.001 92.3 < 0.1 9 3 4.70 1.41~8.00 0.005 90.2 < 0.1 18~36 3 3.88 2.67~5.10 < 0.001 52.9 0.12 精料补饲水平
Concentrate
supplementation
level/(kg·d−1)0.1 3 4.26 1.76~6.76 0.001 92.6 < 0.1 0.15 3 4.87 3.64~6.11 < 0.001 63.7 0.064 0.2~0.4 6 11.74 7.22~16.26 < 0.001 98.3 < 0.1 补饲周期
Supplemental
feeding days/d60~90 10 5.64 3.42~7.86 < 0.001 97.6 < 0.1 180~210 4 12.69 3.94~21.45 < 0.001 98.6 < 0.1 Meta分析结果异质性较大(I 2 > 50%,P < 0.1),亚组分析后,大部分研究间异质性未得到明显改善,故需对分析结果的稳定性和可信度进行评价。采用Egger线性回归法及Begg线性回归法结合漏斗图检验发表偏倚。Begg’s检验Z = 2.39 (P = 0.017),Egger’s检验t = 2.88 (P = 0.008),表示平均日增重这一指标存在发表偏倚(图3A);Begg’s检验Z = 2.56 (P = 0.010),Egger’s检验t = 1.92 (P = 0.070),表明净增重这一指标存在发表偏倚(图3B)。
![]() 图 3 补饲对藏羊生长性能影响的漏斗图和敏感性分析A:补饲对藏羊平均日增重影响的漏斗图;B:补饲对藏羊净增重影响的漏斗图;C:补饲对藏羊平均日增重影响的敏感性分析;D:补饲对藏羊净增重影响的敏感性分析。图中●表示纳入的研究,虚线表示置信区间,中间的实线表示合并效应量;图5同。估值表示剔除该项研究的合并效应量,虚线表示95%CI。Figure 3. Funnel plot and sensitivity analysis of the effects of supplemental feeding on the growth performance of Tibetan sheepA: funnel plot of the effects of supplemental feeding on the average daily weight gain of Tibetan sheep; B: funnel plot of the effects of supplemental feeding on the net weight gain of Tibetan sheep; C: sensitivity analysis of the effects of supplemental feeding on the average daily weight gain in Tibetan sheep; D: sensitivity analysis of the effects of supplemental feeding on the net weight gain of Tibetan sheep. The ● in Figures indicate the included studies, the dotted line represents the confidence interval and the solid line in the middle represents the pooled effect size. This is applicable for Figures as well. Estimates represent the pooled effect sizes excluding the study, and the dotted line represents the 95%CI.
图 3 补饲对藏羊生长性能影响的漏斗图和敏感性分析A:补饲对藏羊平均日增重影响的漏斗图;B:补饲对藏羊净增重影响的漏斗图;C:补饲对藏羊平均日增重影响的敏感性分析;D:补饲对藏羊净增重影响的敏感性分析。图中●表示纳入的研究,虚线表示置信区间,中间的实线表示合并效应量;图5同。估值表示剔除该项研究的合并效应量,虚线表示95%CI。Figure 3. Funnel plot and sensitivity analysis of the effects of supplemental feeding on the growth performance of Tibetan sheepA: funnel plot of the effects of supplemental feeding on the average daily weight gain of Tibetan sheep; B: funnel plot of the effects of supplemental feeding on the net weight gain of Tibetan sheep; C: sensitivity analysis of the effects of supplemental feeding on the average daily weight gain in Tibetan sheep; D: sensitivity analysis of the effects of supplemental feeding on the net weight gain of Tibetan sheep. The ● in Figures indicate the included studies, the dotted line represents the confidence interval and the solid line in the middle represents the pooled effect size. This is applicable for Figures as well. Estimates represent the pooled effect sizes excluding the study, and the dotted line represents the 95%CI.采用逐一剔除法进行敏感性分析,判断所剔除的文献对总合并效应量的影响,结果显示,删除任何一篇文献对平均日增重、净增重指标的总合并效应量均未产生显著的影响,表明补饲对藏羊生长性能影响的Meta分析结果稳健性较好,合并效应量受单篇文献的影响低(图3C、D)。
2.4 补饲对藏羊屠宰性能的影响
I 2统计检验结果显示不同研究间的异质性较大(表5),选择随机效应模型进行后续Meta分析。与未补饲组相比,补饲组置信区间落在无效线的右侧,没有与无效线相交,表明补饲组的效应量大于未补饲组(图4),即补饲极显著提高藏羊胴体重(SMD = 2.57)、屠宰率(SMD = 1.88)和胴体脂肪含量值(SMD = 1.65) (P < 0.001),显著增加藏羊眼肌面积(SMD = 1.24) (P < 0.05)。
表 5 补饲对藏羊屠宰性能影响的Meta分析结果Table 5. Meta-analysis of the effects of supplemental feeding on the slaughter performance of Tibetan sheep测定指标
Measurement研究数量
Numbers of
research/piece标准化均数差 Standard mean difference 异质性检验 Heterogeneity test 效应量
Effect size置信区间(95%CI)
Confidence intervalP I 2/% P 屠宰率
Dressing percentage7 1.88 1.07~2.69 < 0.001 82.7 < 0.1 胴体重
Weight- adjusted fatness value12 2.57 1.59~3.55 < 0.001 91.6 < 0.1 胴体脂肪含量值
Carcass fat content value6 1.65 0.70~2.60 0.001 87.1 < 0.1 眼肌面积
Eye muscle area6 1.24 0.13~2.35 0.029 91.5 < 0.1 Meta分析结果异质性较大(I 2 > 50%,P < 0.1),由于屠宰性能指标纳入文献数量过少,不宜进行亚组分析,采用Egger线性回归法及Begg线性回归法结合漏斗图检验发表偏倚。Begg’s检验Z = 2.81 (P = 0.005),Egger’s检验t = 3.28 (P = 0.008),表示胴体重这一指标存在发表偏倚(图5A);Begg’s检验Z = 0.90 (P = 0.368),Egger’s检验t = 0.73 (P = 0.499),表示屠宰率这一指标不存在发表偏倚(图5B);Begg′s 检验Z = 0.38 (P = 0.707),Egger’s检验t = 1.46 (P = 0.218),表示胴体脂肪含量值这一指标不存在发表偏倚(图5C);Begg’s检验Z = 0.38 (P = 0.707),Egger’s检验t = 0.04 (P = 0.973),表示眼肌面积这一指标不存在发表偏倚(图5D)。
采用逐一剔除法进行敏感性分析,结果显示,删除任何一篇文献对屠宰性能指标的总合并效应量均未产生显著的影响,表明补饲对藏羊屠宰性能影响的Meta分析结果稳健性较好,合并效应量受单篇文献的影响低(图6)。
![]() 图 6 补饲对藏羊屠宰性能影响的敏感性分析A:胴体重;B:屠宰率;C:胴体脂肪含量值;D:眼肌面积。估值表示剔除该项研究的合并效应量,虚线表示95%CI。Figure 6. Sensitivity analysis of the effects of supplemental feeding on the slaughter performance of Tibetan sheepA: carcass weight; B: dressing percentage; C: GR value; D: eye muscle area. Estimates represent the pooled effect size, excluding the study, and the dotted line represents the 95%CI.
图 6 补饲对藏羊屠宰性能影响的敏感性分析A:胴体重;B:屠宰率;C:胴体脂肪含量值;D:眼肌面积。估值表示剔除该项研究的合并效应量,虚线表示95%CI。Figure 6. Sensitivity analysis of the effects of supplemental feeding on the slaughter performance of Tibetan sheepA: carcass weight; B: dressing percentage; C: GR value; D: eye muscle area. Estimates represent the pooled effect size, excluding the study, and the dotted line represents the 95%CI.2.5 研究局限性
本研究存在一定局限性:1)纳入Meta分析的各研究之间,试验动物品种和年龄、放牧草地牧草种类和品质等存在差异;2)纳入文章数量较少,无法对每一个指标都进行亚组分析;3)各研究间异质性大,通过亚组分析仍未降低部分研究间的异质性。
3. 讨论
高寒牧区藏羊养殖以传统的自然放牧管理为主,受到高原恶劣环境及牧草物候期的严格限制[33]。在枯草期,由于牧草资源短缺且营养价值较低,放牧藏羊营养摄入不足导致生产性能下降,甚至引起品种逐渐退化[34-35],极大影响了高寒牧区藏羊养殖业的健康可持续发展。研究发现补饲青贮饲料可以有效地缓解冬春季藏羊掉膘,且在冷季,牧归后补饲精料可以提高藏羊的日增重[4, 21]。平均日增重、净增重等是衡量动物生长性能的关键指标[36]。全晓毅等[37]研究发现牧草枯黄期羔羊补饲精料,平均日增重较对照组显著提高,能够达到预期的育肥效果。本次Meta分析发现,与传统饲养模式相比,补饲显著提高藏羊的平均日增重和净增重,与上述研究结果一致。补饲提高了藏羊营养物质摄入量,且可以更好地满足其摄入营养的均衡性,从而提高饲料转化率[38],有利于促进藏羊生长发育。亚组分析结果显示,补饲精料提高藏羊平均日增重的效果最突出,补饲效果好于精料与粗饲料混合补饲和补饲粗饲料,这与蒋安等[39]的研究结果一致。一方面,藏羊在放牧时已经采食了一定量的粗饲料,比起富含纤维的粗饲料,牧归后的藏羊更需要富含高能高蛋白的精料。另一方面,相比于粗饲料,精料营养更加全面均衡,适口性更好,能够更好地满足藏羊的生长发育需求[40]。
经Meta分析发现,精料补饲效果随着藏羊月龄的增长而降低,其中6月龄以下藏羊精料补饲的效果最好。这与李平业[35]的研究结果一致,符合羔羊6月龄前生长发育快、饲料报酬高的特点[41]。刁其玉和张蓉[42]研究发现4~6月龄犊牛的日增重大于6~12月龄后备牛,这可能是由于随着年龄的增长,家畜生长激素分泌减少、代谢率下降导致其饲料转化率降低[43-45]。研究表明,提高精料水平使日粮中可消化营养物质增加、营养水平升高,进而能够为家畜提供更多能量,促进蛋白质和脂肪在机体内快速沉积,有助于肌肉发育和体重增加[46]。戴东文等[47]研究发现放牧牦牛补饲精料水平越高,平均日增重越大,经济效益越好。本研究Meta分析结果表明,补饲精料提升藏羊平均日增重的效果与补饲水平及补饲时长有关,补饲精料水平越高、补饲周期越长,补饲效果越好,说明放牧藏羊体重增加潜力较大,与薛瑞等[48]对陕北白绒山羊的研究结果一致。因此,对放牧藏羊进行补饲时可以适当增加精料水平及补饲时长,有利于其更好地发挥生长潜力。然而,过量的精料补饲也可能导致瘤胃酸中毒、代谢紊乱等问题[49]。因此,在制定补饲计划时,需要根据动物生长阶段、补饲周期以及补饲水平等因素进行综合考虑,确保精料补饲水平能够取得最佳的生长效果,同时避免不良影响。
屠宰性能可以反映家畜的生长状况及其在实际生产中的产肉能力[50-51]。胴体重、屠宰率是衡量畜禽屠宰性能的重要指标[52]。眼肌面积和胴体脂肪含量值能评估家畜产肉性能及肉品质。眼肌面积是指家畜第十二胸椎和第十三胸椎处背最长肌的横切面面积,与瘦肉率呈正相关关系,眼肌面积越大,表明家畜瘦肉率越高,胴体质量越好[53-55]。胴体脂肪含量值是指12肋骨距离脊柱中心11 cm处脂肪厚度,是胴体脂肪含量的标志,也是评价肉品质优劣的重要因素[56-57]。脂肪含量对肉类的感官品质和加工特性有重要作用,畜禽肉中脂肪的含量会影响到肉制品的质量,适宜的脂肪能够改善肉类或肉制品的嫩度和多汁性[55, 58]。研究表明,冷季补饲精料可刺激器官发育,提高藏羊屠宰性能,且补饲复合微量元素能提高肉品质[23, 25]。王宏博等[22]研究发现对6月龄藏羔羊补饲精料,其胴体重、屠宰率、眼肌面积等均显著提高。本研究分析发现,补饲有助于改善藏羊的屠宰性能及肉品质。与未补饲群体相比,补饲显著提高了藏羊的屠宰率、胴体重、胴体脂肪含量值及眼肌面积,与上述研究结果一致。补饲提高了藏羊摄入的能量水平,有利于营养物质沉积从而提高其屠宰性能[52]。顾玲荣等[59]研究表明,放牧 + 补饲的饲养方式可以提高合作猪的屠宰率、背膘厚和眼肌面积,能够相对增加合作猪的产肉性能,同时不会使脂肪过度沉积。本研究分析结果显示补饲增加了藏羊胴体脂肪含量值和眼肌面积,说明放牧 + 补饲的饲养方式在提高藏羊屠宰性能的同时,能防止脂肪过度沉积。这可能是因为在放牧条件下,藏羊摄入的营养物质有限,无法满足其能量需求,补饲可以提高其营养物质摄入量,增加藏羊胴体脂肪含量,同时放牧 + 补饲的饲养方式又不会减少其必要的运动使脂肪过度沉积。放牧 + 补饲的饲养方式不仅可以提高藏羊屠宰性能,还可以保证藏羊肉品质,能较好地保持藏羊肉“高蛋白,低脂肪”的特性。
试验动物品种、年龄、放牧草地牧草种类和品质、补饲饲料类型、补饲水平及试验周期差异等均可能是本研究异质性的来源,较高的异质性并未影响本次Meta分析的最终结果。在进行Meta分析过程中,动物试验本身特性决定了各研究间会出现较高的异质性,不会影响分析结果的可信度[60-61]。
本研究在Meta层面上分析并证实了补饲对藏羊生长性能和屠宰性能的改善作用。结果表明,精料补饲提升藏羊平均日增重的效果最突出,且补饲效果随藏羊月龄的增加而降低,随精料补饲水平及补饲周期的增加而升高,对6月龄以下藏羊补饲精料效果最好。分析结果可为高牧区藏羊养殖在枯草期的饲养管理提供数据支持。
参考文献
[1] 赵怀志, 杨炀, 张嘉乐, 李江杰, 赵川德, 石岩, 刘同先. 豌豆蚜虫龄期特征及鉴别. 应用昆虫学报, 2021, 58(3): 747-754. ZHAO H Z, YANG Y, ZHANG J L, LI J J, ZHAO C D, SHI Y, LIU T X. Morphological characteristics for distinguishing the instars of Acyrthosiphon pisum. Chinese Journal of Appied Entomology, 2021, 58(3): 747-754.
[2] 薛皎亮, 谢映平, 刘计权, 王金胜, 冀卫荣. 鞘蛾危害后诱导华北落叶松体内化学物质变化的研究. 林业科学, 2000(4): 46-50. doi: 10.3321/j.issn:1001-7488.2000.04.009 XUE J L, XIE Y P, LIU J Q, WANG J S, JI W R. A study on the variation of some chemical substances in the body of larch induced by the damace from the larch case-bearer. Scientia Silvae Sinicae, 2000(4): 46-50. doi: 10.3321/j.issn:1001-7488.2000.04.009
[3] 邵娅, 王森山, 叶超. 单宁酸对红、绿色型豌豆蚜生长发育及繁殖的影响. 草地学报, 2017, 25(4): 866-870. SHAO Y, WANG S S, YE C. Effects of tannic acid on growth and reproduction of red and green pea aphid. Acta Agrestia Sinica, 2017, 25(4): 866-870.
[4] 陆宴辉, 李晓慧, 薛文杰, 杨海燕, 刘洋, 王峰, 余月书, 杨益众. 4种生化物质对棉蚜实验种群增长的影响. 扬州大学学报, 2005(3): 83-87. LU Y H, LI X H, XUE W J, YANG H Y, LIU Y, WANG F, YU Y S, YANG Y Z. Impacts of four biochemicals on population development of Aphis gossypii glover. Journal of Yangzhou University, 2005(3): 83-87.
[5] 陈巨莲, 倪汉祥, 孙京瑞, 程登发. 小麦几种主要次生物质对麦长管蚜几种酶活力的影响. 昆虫学报, 2003(2): 144-149. doi: 10.3321/j.issn:0454-6296.2003.02.003 CHEN J L, NI H X, SUN J R, CHENG D F. Effects of major secondary chemicals of wheat plants on enzyme activity in Sitobion avenae. Acta Entomologica Sinica, 2003(2): 144-149. doi: 10.3321/j.issn:0454-6296.2003.02.003
[6] 达丽婷. 不同苜蓿品种对豌豆蚜消化吸收的影响. 兰州: 甘肃农业大学硕士学位论文, 2015. DA L T. The effect of different alfalfa varieties on the digestion and absorption of pea aphid. Master Thesis. Lanzhou: Gansu Agricultural University, 2015.
[7] DAVIS G K. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biology, 2010, 8(2):e1000313.
[8] 刘长莉, 卢利霞, 许艳丽, 杨鹏程, 崔峰. 灰飞虱唾液腺三大解毒酶家族的转录组分析. 昆虫学报, 2013, 56(12): 1509-1515. doi: 10.16380/j.kcxb.2013.12.006 LIU C L, LU L X, XU Y L, YANG P C, CUI F. Transcriptomic analysis of three detoxification enzyme families in the salivary glands of the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae). Acta Entomologica Sinica, 2013, 56(12): 1509-1515. doi: 10.16380/j.kcxb.2013.12.006
[9] NENA P, JOHN V, THOMAS V L. The role of glutathione- S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Current Opinion in Insect Science, 2018, 27(27): 97-102.
[10] 王瑞龙, 孙玉林, 梁笑婷, 宋圆圆, 苏贻娟, 朱克岩, 曾任森. 6种植物次生物质对斜纹夜蛾解毒酶活性的影响. 生态学报, 2012, 32(16): 5191-5198. doi: 10.5846/stxb201203060302 WANG R L, SUN Y L, LIANG X T, SONG Y Y, SU Y J, ZHU K Y, ZENG R S. Effects of six plant secondary metabolites on activities of detoxification enzymes in Spodoptera litura. Acta Ecologica Sinica, 2012, 32(16): 5191-5198. doi: 10.5846/stxb201203060302
[11] WANG Y Y, HUANG X B, CHANG B H, ZHANG Z H. The survival, growth, and detoxifying enzyme activities of grasshoppers Oedaleus asiaticus (Orthoptera: Acrididae) exposed to toxic rutin. Applied Entomology and Zoology, 2020, 55(4): 385-393. doi: 10.1007/s13355-020-00694-7
[12] HUANG X B, MA J C, QIN X H, TU X B, CAO G C, WANG G J, NONG X Q, ZHANG Z H. Biology, physiology and gene expression of grasshopper Oedaleus asiaticus exposed to diet stress from plant secondary compounds. Scientific Reports, 2017, 7(1): 1-9. doi: 10.1038/s41598-016-0028-x
[13] CAI Q N, HAN Y, CAO Y Z, HU Y, ZHAO X, BI J L. Detoxification of gramine by the cereal aphid Sitobion avenae. Journal of Chemical Ecology, 2009, 35(3): 320-325. doi: 10.1007/s10886-009-9603-y
[14] 黄敏燕, 李雪峰. 植物次生物质对斜纹夜蛾解毒酶活性的影响. 基因组学与应用生物学, 2018, 37(8): 3495-3502. doi: 10.13417/j.gab.037.003495 HUANG M Y, LI X F. Effects of plant secondary metabolite on detoxification enzyme activity of Spodoptera litura. Genomics and Applied Biology, 2018, 37(8): 3495-3502. doi: 10.13417/j.gab.037.003495
[15] XU Z B, ZOU X P, ZHANG N, FENG Q L, ZHANG S C. Detoxification of insecticides, allechemicals and heavy metals by glutathione- S-transferase SlGSTE1 in the gut of Spodoptera litura. Insect Science, 2015, 22(4): 503-511. doi: 10.1111/1744-7917.12142
[16] YANG J, SUN X Q, YAN S Y, PAN W J, ZHANG M X, CAI Q N. Interaction of ferulic acid with glutathione- S-Transferase and carboxylesterase genes in the brown planthopper, Nilaparvata lugens. Journal of Chemical Ecology, 2017, 43(7): 693-702. doi: 10.1007/s10886-017-0859-3
[17] ALI M S, LWANAGA M, KAWASAKI H. Ecdysone-responsive transcriptional regulation determines the temporal expression of cuticular protein genes in wing discs of Bombyx mori. Gene, 2013, 512(2): 337-347. doi: 10.1016/j.gene.2012.09.126
[18] CHAO Y, YI D J, XIN A, LI Y, FENG S, NIU J Z, WANG J J. Effects of RNAi-based silencing of chitin synthase gene on moulting and fecundity in pea aphids ( Acyrthosiphon pisum). Scientific Reports, 2019, 9(1): 1-10. doi: 10.1038/s41598-018-37186-2
[19] 张育霞, 张廷伟, 史历, 袁月, 刘长仲. 外源褪黑素对苜蓿蚜生长发育和繁殖的影响. 生态学杂志, 2023, 42(5): 1150-1154. ZHANG Y X, ZHANG T W, SHI L, YUAN Y, LIU C Z. Effects of exogenous melatonin on the growth and reproduction of Aphis craccivora. Chiness Journal of Ecology, 2023, 42(5): 1150-1154.
[20] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods, 2001, 25(4): 8-10.
[21] 李路莎, 袁郁斐, 武磊, 陈敏. 不同寄主植物对美国白蛾幼虫取食行为及解毒酶活性的影响. 昆虫学报, 2018, 61(2): 232-239. doi: 10.16380/j.kcxb.2018.02.010 LI L S, YUAN Y F, WU L, CHEN M. Effects of host plants on the feeding behavior and detoxification enzyme activities in Hyphantria cunea larvae. Acta Entomologica Sinica, 2018, 61(2): 232-239. doi: 10.16380/j.kcxb.2018.02.010
[22] FEENY P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology, 1970, 51(4): 565-581. doi: 10.2307/1934037
[23] CHEN H, LIU J, CUI K, LU Q, WANG C, WU H X, YANG Z X, DING W F, SHAO S X, WANG H Y, LING X F, KIRST K J, CHEN X M. Molecular mechanisms of tannin accumulation in rhus galls and genes involved in plant-insect interactions. Scientific Reports, 2018, 8(1): 1-12.
[24] LAURENCE D, DAVID J P, GALLET C. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology & Evolution, 2007, 22(6): 298-307.
[25] YUAN Y F, LI L S, ZHAO J F, CHEN M. Effect of tannic acid on nutrition and activities of detoxification enzymes and acetylcholinesterase of the fall webworm (Lepidoptera: Arctiidae). Journal of Insect Science, 2020, 20(1): 1-7. doi: 10.1093/jisesa/iez115
[26] 董钧锋, 张继红, 王琛柱. 植物次生物质对烟青虫和棉铃虫食物利用及中肠解毒酶活性的影响. 昆虫学报, 2002(3): 296-300. doi: 10.3321/j.issn:0454-6296.2002.03.003 DONG J F, ZHANG J H, WANG C Z. Effects of plant allelochemicals on nutritional utilization and detoxication enzyme activities in two Helicoverpa species. Acta Entomologica Sinica, 2002(3): 296-300. doi: 10.3321/j.issn:0454-6296.2002.03.003
[27] 陈澄宇, 康志娇, 史雪岩, 高希武. 昆虫对植物次生物质的代谢适应机制及其对昆虫抗药性的意义. 昆虫学报, 2015, 58(10): 1126-1139. doi: 10.16380/j.kcxb.2015.10.011 CHEN C Y, KANG Z J, SHI X Y, GAO X W. Metabolic adaptation mechanisms of insects to plant secondary metabolites and their implications for insecticide resistance of insects. Acta Entomologica Sinica, 2015, 58(10): 1126-1139. doi: 10.16380/j.kcxb.2015.10.011
[28] 张秀波, 汤方, 刘玉升, 高希武. 单宁酸对杨小舟蛾谷胱甘肽- S-转移酶活性的诱导. 应用昆虫学报, 2009, 46(4): 579-584. doi: 10.3969/j.issn.0452-8255.2009.04.016 ZHANG X B, TANG F, LIU Y S, GAO X W. Induction of glutathione- S-transferases by tannic acid in Micromelalopha troglodyte. Chinese Journal of Applied Entomology, 2009, 46(4): 579-584. doi: 10.3969/j.issn.0452-8255.2009.04.016
[29] TANG F, TU H Z, SHANG Q L, GAO X W, LIANG P. Molecular cloning and characterization of five glutathione- S-transferase genes and promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and their response to tannic acid stress. Insects, 2020, 11(6): 339-355. doi: 10.3390/insects11060339
[30] 刘佳妮, 黄鹤平, 华金珠, 张瑜瑜, 姚丽媛. 烟碱对马铃薯块茎蛾幼虫保护酶和解毒酶的影响. 贵州农业科学, 2015, 43(3): 78-81. doi: 10.3969/j.issn.1001-3601.2015.03.019 LIU J N. HUANG H P, HUA J Z, ZHANG Y Y, YAO Y L. Effects of nicotine on protective and detoxifying enzymes of Phthorimaea opercuella larvae. Guizhou Agricultural Sciences, 2015, 43(3): 78-81. doi: 10.3969/j.issn.1001-3601.2015.03.019
[31] CHEN S, MOHAMMED E A E , DING C H, LI Z F, WANG J. Plant allelochemicals affect tolerance of polyphagous lepidopteran pest Helicoverpa armigera (Hübner) against insecticides. Pesticide Biochemistry and Physiology, 2019, 154 (2): 32-38.
[32] LI Q L, SUN Z X, SHI Q, WANG R M, XU C C, WANG H H, SONG Y Y, ZENG R S. RNA-Seq analyses of midgut and fat body tissues reveal the molecular mechanism underlying Spodoptera litura resistance to tomatine. Frontiers in Physiology, 2019, 10: 1-12.
[33] LOREN J R V, FLOR E A, GARY W F. Genomics of lepidoptera saliva reveals function in herbivory. Current Opinion in Insect Science, 2017, 19(19): 61-69.
[34] 袁星星, 董少奇, 王鑫辉, 郭线茹, 王高平, 李为争, 张利娟, 赵曼. 桃蛀螟细胞色素P450基因 CYP4G113时空表达及功能研究. 中国生物防治学报, 2022, 38(1): 196-204. YUAN X X, DONG S Q, WANG X H, GUO X R, WANG G P, LI W Z, ZHANG L J, ZHAO M. Spatio-temporal expression and function of cytochrome P450 Gene CYP4G113 in Conogethes punctiferalis. Chinese Journal of Biological Control, 2022, 38(1): 196-204.
[35] 张勇, 范佳, 赵兴延, 刘勇, 孙京瑞, Frederic F, 陈巨莲. 植物防御信号物质JA/SA对桃蚜解毒酶谷胱甘肽- S-转移酶及唾液腺基因 C002表达诱导反应. 中国科学:生命科学, 2016, 46(5): 665-672. ZHANG Y, FAN J, ZHAO X Y, LIU Y, SUN J R, FREDERIC F, CHEN J L. Effects of plant defense signal molecules jasmonic acid and salicylic acid on the expression of detoxification enzyme glutathione- S-transferases and salivary protein C002 in Myzus persicae. Scientia Sinica (Vitae), 2016, 46(5): 665-672.
[36] TANG F, ZHANG X B, LIU Y S, GAO X W, LIU N N. In vitro inhibition of glutathione- S-transferases by several insecticides and allelochemicals in two moth species. International Journal of Pest Management, 2014, 60(1): 33-38. doi: 10.1080/09670874.2014.894216
[37] 潘忠玉. 3种次生代谢物对美国白蛾幼虫生长发育及解毒酶活性的影响. 北京: 北京林业大学硕士学位论文, 2020. PAN Z Y. Effects of three secondary metabolites on the growth and development and detoxification enzyme activities in Hyphantria cunea. Master Thesis. Beijing: Beijing Forestry University, 2020.
[38] DAVID J P, STRODE C, VONTAS J, NIKOU D, VAUGHAN A, PIGNATELLI P M, LOUIS C, HEMINGWAY J, RANSON H. The Anopheles gambiae detoxification chip: a highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(11): 4080-4084.
-
图 1 豌豆蚜取食单宁酸48 h头部GST基因表达量的变化
A:上调基因;B:下调基因;C:无显著差异基因。下图同。
Figure 1. Changes in GST genes expression in the head of pea aphids treated with tannic acid for 48 hours
A: upregulated genes; B: downregulated genes; C: genes which were not significantly upregulated or downregulated. **, P < 0.01; *, P < 0.05; ns, P > 0.05. This is applicable for the following figures as well.
表 1 豌豆蚜GST基因qRT-PCR引物序列
Table 1 Primer sequence from qRT-PCR for the GST genes of pea aphids
Contig名
Contig ID引物序列(5′-3′)
Primer sequence (5′-3′)ApGSTomega F-CCGCATGACACAGTGTTCAT
R-GCAAAGGCGGTTCTGGATATApGST101 F-CACTTTGGAACTGCTGCCTT
R-TTGTCTGTGCGGCCTTAAACApGSTthet F-TTCACAGCCGAGTTTGAAGC
R-TGTATGCTGCCACTCAAGGTApGST102 F-CGACGGTGGAAAAGACGATC
R-CATTGAATCACCGGCTGTCCApGST2 F-TTACTACACTCCTGGCAGCC
R-GGCTCTCCCACAAAACCAAGApGST103 F-TACTTCGATCCCATGAGCCC
R-TCCACCGTATTCTCGAACCAApGST104 F-TACTACACTCCTGGCAGTCC
R-ATAATTGCACGGCTCTCCCAApGSTD701 F-GACAACCACGGTCCCATCAT
R-AAACAAGTTCACCGTCCTGCApGSTD6 F-CGATCCTTTCAACCCGCAAT
R-ATGATCGTCTTGTCCACCGTApGST3 F-CCTGGGCAGTCGGAAAGATA
R-GCACTACCGTAATAATCCGCCApGST105 F-CGGTACTAGAGGACGGTGAC
R-GCCGGATACAAGGTTCTGCTApGSTD5 F-CGCCATGCAGATCCGTTATG
R-AGGGAACAGCGGACTATTGTApGSTCD F-TTTGTTGTGTGTCCCTGCTG
R-TTTATCTACTGCCGCCATGCApGST107 F-GCCGTTTTGTTGACTCTCGA
R-CTGTGCTTGAAGTTTCGGGTApGSTD702 F-TTCAGACCGCGGTGATTTTG
R-AAGGGTCGATATGGAAGCGAApGST108 F-AATCCAGTGTTCGAATGTTATGC
R-AATTTCTCCGCCTCCTTTGCApGST109 F-CTGAAAGTACTCGCAATGGC
R-ATTGAGATGAGCCCTTCTGACTApGSTX1 F-GACGTACTTCAACCTGACAGC
R-GATTTCCAACACCGGCACTTApGSTX3 F-TCAACTTCACTGGACTGGGC
R-TTCCCAATCATCGCTACCAGApGSTX4 F-ATGGCCGTGTACAAACTCAC
R-TAACGACTGATGGCTGCTGAApGSTX5 F-ACTTCAACTTCACTGCTTTGGG
R-ACCGTCGATTTCCAACAACGApGSTX6 F-ACTTCAACATCACTGCTCTGG
R-GCCGTCAATTTCCAAAACAGG -
[1] 赵怀志, 杨炀, 张嘉乐, 李江杰, 赵川德, 石岩, 刘同先. 豌豆蚜虫龄期特征及鉴别. 应用昆虫学报, 2021, 58(3): 747-754. ZHAO H Z, YANG Y, ZHANG J L, LI J J, ZHAO C D, SHI Y, LIU T X. Morphological characteristics for distinguishing the instars of Acyrthosiphon pisum. Chinese Journal of Appied Entomology, 2021, 58(3): 747-754.
[2] 薛皎亮, 谢映平, 刘计权, 王金胜, 冀卫荣. 鞘蛾危害后诱导华北落叶松体内化学物质变化的研究. 林业科学, 2000(4): 46-50. doi: 10.3321/j.issn:1001-7488.2000.04.009 XUE J L, XIE Y P, LIU J Q, WANG J S, JI W R. A study on the variation of some chemical substances in the body of larch induced by the damace from the larch case-bearer. Scientia Silvae Sinicae, 2000(4): 46-50. doi: 10.3321/j.issn:1001-7488.2000.04.009
[3] 邵娅, 王森山, 叶超. 单宁酸对红、绿色型豌豆蚜生长发育及繁殖的影响. 草地学报, 2017, 25(4): 866-870. SHAO Y, WANG S S, YE C. Effects of tannic acid on growth and reproduction of red and green pea aphid. Acta Agrestia Sinica, 2017, 25(4): 866-870.
[4] 陆宴辉, 李晓慧, 薛文杰, 杨海燕, 刘洋, 王峰, 余月书, 杨益众. 4种生化物质对棉蚜实验种群增长的影响. 扬州大学学报, 2005(3): 83-87. LU Y H, LI X H, XUE W J, YANG H Y, LIU Y, WANG F, YU Y S, YANG Y Z. Impacts of four biochemicals on population development of Aphis gossypii glover. Journal of Yangzhou University, 2005(3): 83-87.
[5] 陈巨莲, 倪汉祥, 孙京瑞, 程登发. 小麦几种主要次生物质对麦长管蚜几种酶活力的影响. 昆虫学报, 2003(2): 144-149. doi: 10.3321/j.issn:0454-6296.2003.02.003 CHEN J L, NI H X, SUN J R, CHENG D F. Effects of major secondary chemicals of wheat plants on enzyme activity in Sitobion avenae. Acta Entomologica Sinica, 2003(2): 144-149. doi: 10.3321/j.issn:0454-6296.2003.02.003
[6] 达丽婷. 不同苜蓿品种对豌豆蚜消化吸收的影响. 兰州: 甘肃农业大学硕士学位论文, 2015. DA L T. The effect of different alfalfa varieties on the digestion and absorption of pea aphid. Master Thesis. Lanzhou: Gansu Agricultural University, 2015.
[7] DAVIS G K. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biology, 2010, 8(2):e1000313.
[8] 刘长莉, 卢利霞, 许艳丽, 杨鹏程, 崔峰. 灰飞虱唾液腺三大解毒酶家族的转录组分析. 昆虫学报, 2013, 56(12): 1509-1515. doi: 10.16380/j.kcxb.2013.12.006 LIU C L, LU L X, XU Y L, YANG P C, CUI F. Transcriptomic analysis of three detoxification enzyme families in the salivary glands of the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae). Acta Entomologica Sinica, 2013, 56(12): 1509-1515. doi: 10.16380/j.kcxb.2013.12.006
[9] NENA P, JOHN V, THOMAS V L. The role of glutathione- S-transferases (GSTs) in insecticide resistance in crop pests and disease vectors. Current Opinion in Insect Science, 2018, 27(27): 97-102.
[10] 王瑞龙, 孙玉林, 梁笑婷, 宋圆圆, 苏贻娟, 朱克岩, 曾任森. 6种植物次生物质对斜纹夜蛾解毒酶活性的影响. 生态学报, 2012, 32(16): 5191-5198. doi: 10.5846/stxb201203060302 WANG R L, SUN Y L, LIANG X T, SONG Y Y, SU Y J, ZHU K Y, ZENG R S. Effects of six plant secondary metabolites on activities of detoxification enzymes in Spodoptera litura. Acta Ecologica Sinica, 2012, 32(16): 5191-5198. doi: 10.5846/stxb201203060302
[11] WANG Y Y, HUANG X B, CHANG B H, ZHANG Z H. The survival, growth, and detoxifying enzyme activities of grasshoppers Oedaleus asiaticus (Orthoptera: Acrididae) exposed to toxic rutin. Applied Entomology and Zoology, 2020, 55(4): 385-393. doi: 10.1007/s13355-020-00694-7
[12] HUANG X B, MA J C, QIN X H, TU X B, CAO G C, WANG G J, NONG X Q, ZHANG Z H. Biology, physiology and gene expression of grasshopper Oedaleus asiaticus exposed to diet stress from plant secondary compounds. Scientific Reports, 2017, 7(1): 1-9. doi: 10.1038/s41598-016-0028-x
[13] CAI Q N, HAN Y, CAO Y Z, HU Y, ZHAO X, BI J L. Detoxification of gramine by the cereal aphid Sitobion avenae. Journal of Chemical Ecology, 2009, 35(3): 320-325. doi: 10.1007/s10886-009-9603-y
[14] 黄敏燕, 李雪峰. 植物次生物质对斜纹夜蛾解毒酶活性的影响. 基因组学与应用生物学, 2018, 37(8): 3495-3502. doi: 10.13417/j.gab.037.003495 HUANG M Y, LI X F. Effects of plant secondary metabolite on detoxification enzyme activity of Spodoptera litura. Genomics and Applied Biology, 2018, 37(8): 3495-3502. doi: 10.13417/j.gab.037.003495
[15] XU Z B, ZOU X P, ZHANG N, FENG Q L, ZHANG S C. Detoxification of insecticides, allechemicals and heavy metals by glutathione- S-transferase SlGSTE1 in the gut of Spodoptera litura. Insect Science, 2015, 22(4): 503-511. doi: 10.1111/1744-7917.12142
[16] YANG J, SUN X Q, YAN S Y, PAN W J, ZHANG M X, CAI Q N. Interaction of ferulic acid with glutathione- S-Transferase and carboxylesterase genes in the brown planthopper, Nilaparvata lugens. Journal of Chemical Ecology, 2017, 43(7): 693-702. doi: 10.1007/s10886-017-0859-3
[17] ALI M S, LWANAGA M, KAWASAKI H. Ecdysone-responsive transcriptional regulation determines the temporal expression of cuticular protein genes in wing discs of Bombyx mori. Gene, 2013, 512(2): 337-347. doi: 10.1016/j.gene.2012.09.126
[18] CHAO Y, YI D J, XIN A, LI Y, FENG S, NIU J Z, WANG J J. Effects of RNAi-based silencing of chitin synthase gene on moulting and fecundity in pea aphids ( Acyrthosiphon pisum). Scientific Reports, 2019, 9(1): 1-10. doi: 10.1038/s41598-018-37186-2
[19] 张育霞, 张廷伟, 史历, 袁月, 刘长仲. 外源褪黑素对苜蓿蚜生长发育和繁殖的影响. 生态学杂志, 2023, 42(5): 1150-1154. ZHANG Y X, ZHANG T W, SHI L, YUAN Y, LIU C Z. Effects of exogenous melatonin on the growth and reproduction of Aphis craccivora. Chiness Journal of Ecology, 2023, 42(5): 1150-1154.
[20] LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods, 2001, 25(4): 8-10.
[21] 李路莎, 袁郁斐, 武磊, 陈敏. 不同寄主植物对美国白蛾幼虫取食行为及解毒酶活性的影响. 昆虫学报, 2018, 61(2): 232-239. doi: 10.16380/j.kcxb.2018.02.010 LI L S, YUAN Y F, WU L, CHEN M. Effects of host plants on the feeding behavior and detoxification enzyme activities in Hyphantria cunea larvae. Acta Entomologica Sinica, 2018, 61(2): 232-239. doi: 10.16380/j.kcxb.2018.02.010
[22] FEENY P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology, 1970, 51(4): 565-581. doi: 10.2307/1934037
[23] CHEN H, LIU J, CUI K, LU Q, WANG C, WU H X, YANG Z X, DING W F, SHAO S X, WANG H Y, LING X F, KIRST K J, CHEN X M. Molecular mechanisms of tannin accumulation in rhus galls and genes involved in plant-insect interactions. Scientific Reports, 2018, 8(1): 1-12.
[24] LAURENCE D, DAVID J P, GALLET C. The evolutionary ecology of insect resistance to plant chemicals. Trends in Ecology & Evolution, 2007, 22(6): 298-307.
[25] YUAN Y F, LI L S, ZHAO J F, CHEN M. Effect of tannic acid on nutrition and activities of detoxification enzymes and acetylcholinesterase of the fall webworm (Lepidoptera: Arctiidae). Journal of Insect Science, 2020, 20(1): 1-7. doi: 10.1093/jisesa/iez115
[26] 董钧锋, 张继红, 王琛柱. 植物次生物质对烟青虫和棉铃虫食物利用及中肠解毒酶活性的影响. 昆虫学报, 2002(3): 296-300. doi: 10.3321/j.issn:0454-6296.2002.03.003 DONG J F, ZHANG J H, WANG C Z. Effects of plant allelochemicals on nutritional utilization and detoxication enzyme activities in two Helicoverpa species. Acta Entomologica Sinica, 2002(3): 296-300. doi: 10.3321/j.issn:0454-6296.2002.03.003
[27] 陈澄宇, 康志娇, 史雪岩, 高希武. 昆虫对植物次生物质的代谢适应机制及其对昆虫抗药性的意义. 昆虫学报, 2015, 58(10): 1126-1139. doi: 10.16380/j.kcxb.2015.10.011 CHEN C Y, KANG Z J, SHI X Y, GAO X W. Metabolic adaptation mechanisms of insects to plant secondary metabolites and their implications for insecticide resistance of insects. Acta Entomologica Sinica, 2015, 58(10): 1126-1139. doi: 10.16380/j.kcxb.2015.10.011
[28] 张秀波, 汤方, 刘玉升, 高希武. 单宁酸对杨小舟蛾谷胱甘肽- S-转移酶活性的诱导. 应用昆虫学报, 2009, 46(4): 579-584. doi: 10.3969/j.issn.0452-8255.2009.04.016 ZHANG X B, TANG F, LIU Y S, GAO X W. Induction of glutathione- S-transferases by tannic acid in Micromelalopha troglodyte. Chinese Journal of Applied Entomology, 2009, 46(4): 579-584. doi: 10.3969/j.issn.0452-8255.2009.04.016
[29] TANG F, TU H Z, SHANG Q L, GAO X W, LIANG P. Molecular cloning and characterization of five glutathione- S-transferase genes and promoters from Micromelalopha troglodyta (Graeser) (Lepidoptera: Notodontidae) and their response to tannic acid stress. Insects, 2020, 11(6): 339-355. doi: 10.3390/insects11060339
[30] 刘佳妮, 黄鹤平, 华金珠, 张瑜瑜, 姚丽媛. 烟碱对马铃薯块茎蛾幼虫保护酶和解毒酶的影响. 贵州农业科学, 2015, 43(3): 78-81. doi: 10.3969/j.issn.1001-3601.2015.03.019 LIU J N. HUANG H P, HUA J Z, ZHANG Y Y, YAO Y L. Effects of nicotine on protective and detoxifying enzymes of Phthorimaea opercuella larvae. Guizhou Agricultural Sciences, 2015, 43(3): 78-81. doi: 10.3969/j.issn.1001-3601.2015.03.019
[31] CHEN S, MOHAMMED E A E , DING C H, LI Z F, WANG J. Plant allelochemicals affect tolerance of polyphagous lepidopteran pest Helicoverpa armigera (Hübner) against insecticides. Pesticide Biochemistry and Physiology, 2019, 154 (2): 32-38.
[32] LI Q L, SUN Z X, SHI Q, WANG R M, XU C C, WANG H H, SONG Y Y, ZENG R S. RNA-Seq analyses of midgut and fat body tissues reveal the molecular mechanism underlying Spodoptera litura resistance to tomatine. Frontiers in Physiology, 2019, 10: 1-12.
[33] LOREN J R V, FLOR E A, GARY W F. Genomics of lepidoptera saliva reveals function in herbivory. Current Opinion in Insect Science, 2017, 19(19): 61-69.
[34] 袁星星, 董少奇, 王鑫辉, 郭线茹, 王高平, 李为争, 张利娟, 赵曼. 桃蛀螟细胞色素P450基因 CYP4G113时空表达及功能研究. 中国生物防治学报, 2022, 38(1): 196-204. YUAN X X, DONG S Q, WANG X H, GUO X R, WANG G P, LI W Z, ZHANG L J, ZHAO M. Spatio-temporal expression and function of cytochrome P450 Gene CYP4G113 in Conogethes punctiferalis. Chinese Journal of Biological Control, 2022, 38(1): 196-204.
[35] 张勇, 范佳, 赵兴延, 刘勇, 孙京瑞, Frederic F, 陈巨莲. 植物防御信号物质JA/SA对桃蚜解毒酶谷胱甘肽- S-转移酶及唾液腺基因 C002表达诱导反应. 中国科学:生命科学, 2016, 46(5): 665-672. ZHANG Y, FAN J, ZHAO X Y, LIU Y, SUN J R, FREDERIC F, CHEN J L. Effects of plant defense signal molecules jasmonic acid and salicylic acid on the expression of detoxification enzyme glutathione- S-transferases and salivary protein C002 in Myzus persicae. Scientia Sinica (Vitae), 2016, 46(5): 665-672.
[36] TANG F, ZHANG X B, LIU Y S, GAO X W, LIU N N. In vitro inhibition of glutathione- S-transferases by several insecticides and allelochemicals in two moth species. International Journal of Pest Management, 2014, 60(1): 33-38. doi: 10.1080/09670874.2014.894216
[37] 潘忠玉. 3种次生代谢物对美国白蛾幼虫生长发育及解毒酶活性的影响. 北京: 北京林业大学硕士学位论文, 2020. PAN Z Y. Effects of three secondary metabolites on the growth and development and detoxification enzyme activities in Hyphantria cunea. Master Thesis. Beijing: Beijing Forestry University, 2020.
[38] DAVID J P, STRODE C, VONTAS J, NIKOU D, VAUGHAN A, PIGNATELLI P M, LOUIS C, HEMINGWAY J, RANSON H. The Anopheles gambiae detoxification chip: a highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(11): 4080-4084.




 下载:
下载: