干旱胁迫对甘草幼苗光合特性及根系吸水的影响
甘草(Glycyrrhiza uralensis)为豆科甘草属多年生草本植物,具有极高的药用价值,是我国干旱、半干旱地区重要的经济和生态作物。土壤水分是限制甘草生长的主要环境因素之一,研究其抗旱适应性表现对甘草资源可持续利用具有重要意义。本研究采用聚乙二醇6000 (PEG-6000)模拟干旱胁迫,研究胀果甘草(G. inflata)和乌拉尔甘草(G. uralensis)幼苗的光合特性和根系吸水能力对干旱胁迫的响应。结果表明:干旱胁迫7 d后胀果甘草和乌拉尔甘草幼苗地上部干重和根系干重均显著降低,其中地上部干重分别降低56.10%、62.50%,地下部干重分别降低16.67%和28.57%,而根冠比均有所增加。干旱胁迫均造成了胀果甘草和乌拉尔甘草幼苗叶片净光合速率(Pn)、蒸腾速率(Tr)和气孔导度(Gs)的降低,与对照相比,其下降幅度分别为41.73%、67.22%,62.21%、75.38%,66.02%、82.60%。干旱胁迫下甘草幼苗荧光特性也被显著抑制(P < 0.05),其中胀果甘草幼苗的光系统Ⅱ潜在活性(Fv/Fo)和最大光能转换效率(Fv/Fm)与对照相比分别降低21.94%和9.09%,乌拉尔甘草幼苗的Fv/Fo和Fv/Fm与对照相比分别降低35.10%和10.39%;胀果甘草和乌拉尔甘草幼苗的光化学淬灭系数(qP)在15% PEG胁迫下分别降低17.65%和27.27%。干旱胁迫降低了两种甘草幼苗叶片相对含水量和叶水势,其中胀果甘草幼苗叶片相对含水量降低11.07%,叶水势降低19.54%;乌拉尔甘草幼苗叶片相对含水量降低13.91%,叶水势降低62.56%。干旱胁迫抑制了两种甘草的根系生长,根表面积和根体积减少但增加了总根长,其中胀果甘草根系显著小于乌拉尔甘草,但胀果甘草干旱胁迫下整株根系水力学导度降幅小于乌拉尔甘草。说明在受到干旱胁迫时,胀果甘草可以保持较高的光合作用优势,同时其根系较小具有更高的根系效率,通过更强的根系吸水能力提高了作物水分利用效率,具有更强的抗旱能力。本研究可为补充完善甘草耐旱性理论提供依据,并为人工栽培及旱生植物资源保护与利用提供策略。
English
-
干旱严重限制农牧业生产并极大地影响着野生植被的生存发展,是最具破坏性的非生物胁迫之一,影响作物生长、品质形成并最终影响产量,同时还会引发一系列环境问题如草原退化、土地荒漠化、水土流失等[1-3]。叶片光合作用是调节植物生长发育最重要的代谢过程,但对环境胁迫非常敏感,尤其是干旱胁迫[4]。干旱条件导致的植物气孔调节能力降低、必需酶和光合色素的合成减少和活性改变、腺苷三磷酸(adenosine triphosphate, ATP)合成受损和光合机构的破坏是降低光合作用的关键因素[5]。气孔关闭通常是对干旱胁迫的第一反应,植物通过气孔来调节光合作用的CO2吸收和蒸腾作用的水分损失[6]。在轻度至中度干旱胁迫下,气孔关闭降低了光合速率导致能量过剩,加强活性氧(reactive oxygen species, ROS)的积累和膜脂质过氧化程度对光系统Ⅱ(PSⅡ)功能造成严重损害[7]。关于干旱胁迫对PSⅡ活性的影响,有相互矛盾的报道,一些研究认为PSⅡ损伤只发生在严重干旱胁迫条件下。他们提出PSⅡ活性的下调、光合电子传递速率ETR的下降甚至PSⅡ的光抑制都是应对干旱胁迫的潜在保护机制[8]。因此,评估光合系统的功能有助于预测作物抵御干旱胁迫的能力。植物进行光合作用的同时伴随着水分蒸散,维持植物体内水分的平衡需要根系和/或冠层感受到环境刺激并通过两者间信号的传递启动整体性的行为才能完成[9-10]。植物的水分运输与调控是保证其生命活动正常进行的重要生理过程,土壤-植物-大气连续体(soil-plant-atmosphere continuum, SPAC)的水流途径是:土壤水分向植物根系移动,由根表面穿过表皮皮层内皮层进入根木质部,沿根茎木质部导管输送到叶片,在叶片胞间空隙汽化,水汽由气孔和角质层扩散到与叶面接触的静空气层,最后通过湍流转移到外界大气[11]。
作为植物水分的吸收器官,根系的数量、大小、干物质和生理状况等可直接影响作物抗旱性的强弱,当干旱胁迫发生时根系迅速反应通过调整自身形态、结构以减少水分损失适应环境变化,同时根系直接接触土壤可以及时感知外界环境变化并向地上部传导逆境根源信号,促使叶片调节气孔从而降低蒸腾作用带来的水分损失[12]。植物在自然选择过程中进化出了多种逆境适应机制,其中根系结构的改变是干旱条件下获取充足水分和养分的关键因素之一[13-14]。干旱胁迫刺激侧根和根毛的形成,并诱导根系的可塑性,增加根系分支的数量,减少侧根直径,改变根系生物量[15-16]。根系水力学导度对干旱敏感,可能控制着整个植物的水力导度和植物活力[17]。前人研究发现干旱胁迫在胁迫初期通过降低紫花苜蓿(Medicago sativa)和高粱(Sorghum bicolor)根系水力学导度和根系活力继而调控其地上部反应,引起光合作用的下降[18]。对小麦(Triticum aestivum)关键生育期进行轻度控水处理可以达到有效提高水分利用效率(water use efficiency, WUE)且不降低产量的目的,同时抗旱性强的小麦品种根系水力学导度优于抗旱性较弱的[19]。忽雪琦等[20]发现玉米(Zea mays)的根系吸水能力的增强能够缓解干旱胁迫造成的叶片水分含量的下降和水势的降低,从而提高玉米幼苗的抗旱性。
此外,研究表明植物基因型对于耐旱性有极大影响[21],且不同品种对干旱胁迫的响应可能存在不同的机理[22]。两类新疆狗牙根(Cynodon dactylon)抗旱基因型对比发现,不抗旱基因型的脂质过氧化高于抗旱基因型,而抗氧化酶活性低于抗旱基因型,同时抗旱基因型中的抗氧化基因表达水平高于不抗旱基因型[23]。赵小强[24]等推测抗旱型玉米株型结构与气孔形态、生理代谢及光合特性相互调节共同构成玉米株型相关耐旱响应机制。
甘草(Glycyrrhiza uralensis)为豆科多年生草本植物,是我国常用的大宗药材之一[25],具有“十方九草”的美称,可见其应用广泛。甘草主要分布在我国干旱及半干旱地区,具有很高的固沙能力,同时还具有耐旱、耐寒、耐盐碱等优良特性,是我国干旱、半干旱地区进行防风固沙、改善土壤结构的重要植物资源[26-27]。《中国药典》收录的甘草为乌拉尔甘草(G. uralensis)、胀果甘草(G. inflata)和光果甘草(G. glabra)根和根茎的干制品。前人研究发现干旱胁迫显著抑制甘草种子发芽率、发芽势及萌发指数[26, 28]。轻度干旱胁迫提高甘草抗氧化酶类活性减轻膜损伤,同时通过积累渗透调节物质来提高植株的吸水和保水能力[29-30];然而重度胁迫造成活性氧代谢平衡破坏加剧膜脂过氧化[30]。关思静等[31]通过转录组分析得出干旱会导致甘草净光合速率、气孔导度、蒸腾速率降低,叶片和根部通过诱导保护酶基因的表达以调节抗氧化酶活性的方式来清除ROS。刘长乐等[32]研究发现适宜的外源植物激素预处理可促进野生乌拉尔甘草的种子发芽。
目前对甘草的抗旱性相关研究主要集中于单一品种的形态建成和抗氧化代谢,而尚未有关于根系吸水能力变化的研究。本研究以两种不同甘草为材料,通过研究叶片净光合速率、蒸腾速率、气孔导度、叶片水势、根系形态特征与根系吸水能力的关系,探讨干旱胁迫下甘草根系吸水的变化机制,为进一步补充完善甘草耐旱性理论提供依据。
1. 材料与方法
1.1 试验材料及试验设计
试验为双因素试验设计,主区为2个甘草品种:胀果甘草和乌拉尔甘草;副区为2个处理:对照组[CK,未添加聚乙二醇(polyethylene glycol 6000, PEG-6000)的Hoagland营养液]和处理组[15%,Hoagland营养液中添加终浓度为15%的PEG-6000]。各处理均设3个独立重复。
挑选健康、饱满的种子用98%浓硫酸浸泡30 min,期间不断搅拌,清水反复冲洗后浸泡在体积比为1 ꞉ 3的30%过氧化氢 ꞉ 纯净水溶液中消毒30 min,用去离子水洗净后,纯水浸泡24 h使种子吸胀,待种子萌发后播种至育苗盘中进行育苗。待甘草苗长出五六片叶子后筛选长势基本一致的植株转移至塑料水培盆中,以纯净水浇灌缓苗培养24 h,更换后采用Hoagland全营养液水培20 d,随后进行干旱胁迫处理。处理组用Hoagland全营养液配制15% PEG-6000溶液模拟干旱胁迫[31],以不加PEG-6000的Hoagland全营养液培养的幼苗为对照组。处理时间均为7 d。待处理时间达到7 d时测定其光合指标,并立即在实验室进行鲜样指标的测定。
1.2 测定项目及方法
1.2.1 根系生长及形态特征的测定
将甘草植株取样后,使用去离子水冲洗干净,并用剪刀分成地上部茎叶和地下部根系两部分,吸水纸吸干水分,称取各部分鲜重;将烘箱温度设置为105 ℃,放入样品杀青30 min;接着将温度调至85 ℃,烘干至恒重,称取干重,进一步计算生物量和根冠比。
形态特征的测定:取甘草完整根系,冲洗根部,用吸水纸吸干幼苗根系的表面水分,均匀铺开,经Epson Perfection V700扫描仪(Seiko Epson, 日本)扫描后,用WinRHIZO PRO STD4800型(Regent, 加拿大)根系图象处理软件分析得出根长、根系表面积、根体积及根平均直径。
1.2.2 光合指标和荧光参数测定
处理7 d后,在09:00-11:00选取各处理植株中部叶片,用Li-6400光合仪测定叶片净光合速率(net photosynthetic rate, Pn)、蒸腾速率(transpiration rate, Tr)、气孔导度(stomatal conductance, Gs)、胞间CO2浓度(intercellular CO2 concentration, Ci),叶室CO2浓度约为380 µmol·mol−1,光合有效辐射约为1100µmol·(m2·s)−1,重复3次。植物暗适应30 min后使用Imaging-PAM (Walz, Effeltrich, Germany)荧光仪测定每个处理植株中部叶片,获取荧光参数指标,重复3次。
1.2.3 叶片相对含水量测定
取0.2 g待测鲜叶,流水冲洗,去离子水清洗后滤纸吸干表面浮水,称鲜重。浸入装有蒸馏水的烧杯,使组织吸水至恒重,称饱和重量;后置于105 ℃烘箱内烘15~20 min,杀死组织,再于80 ℃下烘6~8 h,待达到恒重时,取出冷却,称其干重,计算相对含水量。
相对含水量 = (组织鲜重 − 组织干重)/(组织水分饱和重量 − 组织干重) × 100%。
1.2.4 叶片水势的测定
于上午10:00取各处理从上数第3片完全展开叶,用3005型压力室(Soil Moisture Equipment Crop., Santa Barbara, USA)法[33]测定其叶中部水势。
1.2.5 水力学导度的测定
整株根系水力学导度的测定采用压力室法[33-34]。将一条完整独立的甘草幼苗种子根剪断于根基部,露出约1 cm的中胚轴,根系用硅胶塞密封在钢瓶中,然后塞入压力室中。拧紧之后,以0.1 MPa·s−1的速度缓慢加压至0.2 MPa,径流达到稳定状态后(约1 min),直接以0.9 mm的毛细管吸收径流1 min。之后依次测定在0.3 及0.4 MPa时的水流量,并转化为体积。各压力下根系吸水能力用单位时间内单位根表面积出水量来表示,计算公式为:
$$ L_p=\frac{V}{S\times p\times t}。 $$ 式中:Lp为根系水流导度[m·(s·MPa)−1];V为t (s)时间内通过测试根的水流总体积(m3);S为测试根段的表面积(m2);P为达到出流稳态时的平衡压(即外界所加压力,MPa)。根系水力学导度(root hydraulic conductivity)用单位面积下出水量与所对应的压力关系直线的斜率来表示。
1.3 数据统计与分析
使用Excel 2010和Origin 2022进行数据整理和图形绘制,使用SPSS 27.0软件对数据进行统计分析,进行LSD双因素方差分析和邓肯差异显著性检验,比较品种及干旱胁迫及其交互作用对甘草生长、光合荧光特性及整株根系水导的影响,用平均值和标准差表示测定结果。
2. 结果与分析
2.1 干旱胁迫下甘草幼苗的生长响应及根系构型变化
如表1所列,在正常供水条件下(CK),乌拉尔甘草幼苗生物量显著高于胀果甘草幼苗。在15% PEG胁迫条件下,甘草幼苗生长受到抑制,总干重较CK显著降低,其中胀果和乌拉尔甘草幼苗根干重较CK分别降低16.67%和28.57%,但差异不显著(P > 0.05),地上部干重分别显著降低56.10%和62.50% (P < 0.05)。同时,干旱胁迫导致甘草幼苗的根冠比有所增加。
表 1 干旱胁迫对甘草生物量和根冠比的影响Table 1. Effects of drought stress on biomass and root-shoot ratio of Glycyrrhiza inflata and G. uralensis品种
Variety处理
Treatment地上部鲜重
Shoot fresh
weight/g地上部干重
Shoot dry
weight/g根鲜重
Root fresh
weight/g根干重
Root dry
weight/g总干重
Total dry
weight/g根冠比
Root-shoot
ratio/%胀果甘草
G. inflataCK 2.17 ± 0.21b 0.41 ± 0.14b 0.79 ± 0.10bc 0.06 ± 0.01bc 0.47 ± 0.14b 17.17 ± 5.67ab 15% PEG 0.69 ± 0.06c 0.18 ± 0.03c 0.49 ± 0.12c 0.05 ± 0.00b 0.23 ± 0.03c 26.59 ± 4.83a 乌拉尔甘草
G. uralensisCK 6.04 ± 0.47a 1.12 ± 0.09a 1.96 ± 0.37a 0.14 ± 0.03a 1.27 ± 0.11a 12.61 ± 1.79b 15% PEG 1.06 ± 0.08c 0.42 ± 0.05b 1.16 ± 0.29b 0.10 ± 0.03ab 0.52 ± 0.08b 24.43 ± 4.07a 变异来源
Source of variation品种 Variety (V) *** *** *** *** *** ns 15% PEG (P) *** *** * ns *** ** V × P *** ** ns ns ** ns 同列不同小写字母表示差异显著(P < 0.05)。CK:对照;15% PEG:Hoagland全营养液配制15% PEG-6000溶液模拟干旱胁迫。*、**、***分别表示P < 0.05、0.01、0.001;ns:不显著,P ≥ 0.05。下同。
Different lowercase letters in the same column represent significant difference at the 0.05 level. CK: control; 15% PEG: 15% PEG-6000 solution prepared with Hoagland nutrient solution was used to simulate drought stress. *, **, *** indicate P < 0.05, 0.01, 0.001, respectively; ns: not significant, P ≥ 0.05. This is applicable for the following figures and tables as well.干旱胁迫抑制甘草幼苗根系生长,对总根长和单株总根表面积均有显著影响(P < 0.05) (表2)。干旱胁迫下胀果甘草和乌拉尔甘草幼苗总根长显著升高(P < 0.05),与CK相比分别增加25.22%和19.97%;在干旱胁迫下甘草幼苗单株总根表面积均降低,其中胀果甘草显著降低21.33% (P < 0.05),乌拉尔甘草幼苗显著降低24.04% (P < 0.05)。同时,干旱胁迫抑制胀果甘草与乌拉尔甘草幼苗根系的增粗生长,平均直径降低,与CK相比分别显著降低15.69% 和 16.13% (P < 0.05),根系体积分别降低8.85%和8.15%,无显著差异(P > 0.05)。
表 2 干旱胁迫对两种甘草根系构型的影响Table 2. Effects of drought stress on total root length, total root surface area, root volume, and mean root diameter of Glycyrrhiza inflata and G. uralensis品种
Variety处理
Treatment总根长
Total root length/cm总根表面积
Total root surface area/cm2根体积
Root volume/cm3平均直径
Mean root diameter/mm胀果甘草
G. inflataCK 352.94 ± 8.13d 83.34 ± 1.42b 1.13 ± 0.02a 0.51 ± 0.06b 15% PEG 441.96 ± 11.39c 65.56 ± 8.51c 1.03 ± 0.34a 0.43 ± 0.01c 乌拉尔甘草
G. uralensisCK 523.20 ± 49.21b 118.34 ± 10.69a 1.35 ± 0.54a 0.62 ± 0.03a 15% PEG 627.69 ± 15.01a 89.90 ± 3.32b 1.24 ± 0.02a 0.52 ± 0.01b 变异来源
Source of variation品种 Variety (V) *** *** ns ** 15% PEG (P) *** ** ns ** V × P ns ns ns ns 2.2 干旱胁迫对甘草幼苗叶水势和相对含水量的影响
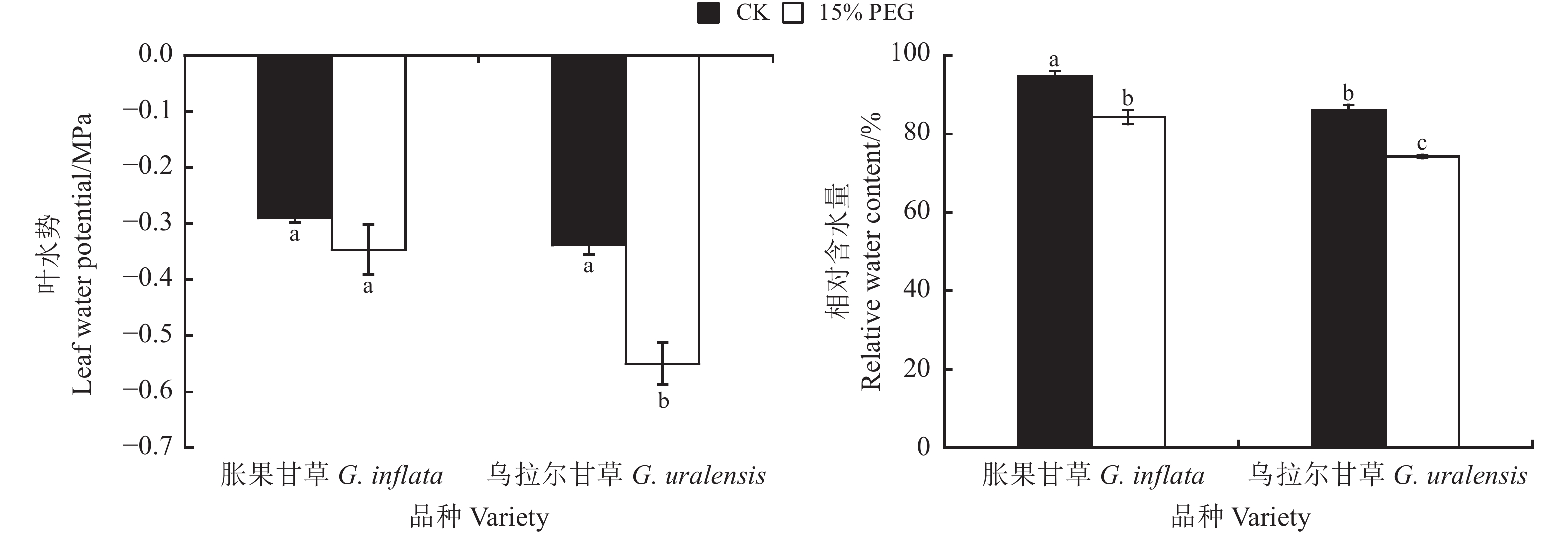
正常水分条件下甘草幼苗叶片相对含水量为80%~95%,叶水势维持在−0.3 MPa左右,其中胀果甘草幼苗叶水势和相对含水量均高于乌拉尔甘草幼苗;15% PEG胁迫处理下,两种甘草幼苗的叶片相对含水量和叶水势均降低,但下降幅度有所不同(图1)。其中胀果甘草幼苗叶片相对含水量显著降低11.07% (P < 0.05),叶水势降低19.54% (P > 0.05);乌拉尔甘草幼苗叶片相对含水量显著降低13.91% (P < 0.05),叶水势显著降低62.56% (P < 0.05),此时两种甘草幼苗处于失水的状态。
![]() 图 1 干旱胁迫下甘草叶片相对含水量和叶水势不同小写字母表示不同处理间差异显著(P < 0.05);下图同。Figure 1. Influence of leaf relative water content and water potential of Glycyrrhiza inflata and G. uralensis on drought stressDifferent lowercase letters indicate significant differences between different treatments at the 0.05 level. This is applicable for the following figures as well.
图 1 干旱胁迫下甘草叶片相对含水量和叶水势不同小写字母表示不同处理间差异显著(P < 0.05);下图同。Figure 1. Influence of leaf relative water content and water potential of Glycyrrhiza inflata and G. uralensis on drought stressDifferent lowercase letters indicate significant differences between different treatments at the 0.05 level. This is applicable for the following figures as well.2.3 干旱胁迫对甘草幼苗光合特性的影响
正常水分处理条件下,胀果甘草和乌拉尔甘草幼苗的Pn分别为3.81和4.33 µmol·(m2·s)−1,干旱胁迫下叶片Pn较CK分别显著下降了41.73%、67.22% (P < 0.05) (图2)。干旱胁迫下,甘草幼苗叶片Gs和Tr与Pn呈同步变化,较CK显著降低(P < 0.05),且乌拉尔甘草的降低幅度高于胀果甘草。胀果甘草和乌拉尔甘草幼苗叶片的Ci在干旱胁迫处理下也表现出显著降低(P < 0.05),分别降低4.43%、19.81%。
干旱胁迫条件下胀果甘草和乌拉尔甘草幼苗的WUE均提高,但是差异不显著(P > 0.05),分别达到CK的1.57和1.36倍,说明干旱胁迫引起了甘草幼苗WUE在一定程度上的提高,同时胀果甘草WUE相比于乌拉尔甘草在干旱胁迫下增幅较大,保持较高值(图2)。
2.4 干旱胁迫对甘草幼苗叶绿素荧光学参数的影响
干旱胁迫显著抑制甘草幼苗荧光特性(P < 0.05) (表3)。干旱胁迫下胀果甘草幼苗初始荧光(minimal fluorescence, Fo)升高且最大荧光(maximum fluorescence, Fm)降低,无显著差异(P > 0.05),而乌拉尔甘草幼苗的Fo较CK显著升高11.11%,Fm显著降低18.42% (P < 0.05)。正常供水条件下,胀果甘草和乌拉尔甘草幼苗的最大光能转换效率(maximal photochemical efficiency, Fv/Fm)均在0.70左右。在15% PEG胁迫处理下,甘草幼苗的光系统Ⅱ潜在活性(potential efficiency of PSⅡ, Fv/Fo)和Fv/Fm均显著下降(P < 0.05),其中胀果甘草幼苗的Fv/Fo和Fv/Fm分别比CK降低21.94%和9.09%,乌拉尔甘草幼苗的Fv/Fo和Fv/Fm分别比CK降低35.10%和10.39%。与CK相比,干旱胁迫下胀果甘草和乌拉尔甘草幼苗的光化学淬灭系数(photochemical quenching coefficient, qP)均显著降低(P < 0.05),分别降低17.65%和27.27%。
表 3 干旱胁迫下甘草叶绿素荧光参数Table 3. Effects of chlorophyⅡ fluorescence parameters of Glycyrrhiza inflata and G. uralensis on drought stress品种
Variety处理
TreatmentFo Fm Fv/Fo Fv/Fm qP 胀果甘草
G. inflataCK 0.089 ± 0.004ab 0.26 ± 0.01c 1.96 ± 0.01b 0.66 ± 0.01b 0.34 ± 0.04a 15% PEG 0.090 ± 0.002ab 0.23 ± 0.02c 1.53 ± 0.17c 0.60 ± 0.03c 0.28 ± 0.01bc 乌拉尔甘草
G. uralensisCK 0.088 ± 0.004b 0.38 ± 0.02a 3.39 ± 0.32a 0.77 ± 0.02a 0.33 ± 0.02ab 15% PEG 0.096 ± 0.002a 0.31 ± 0.01b 2.20 ± 0.03b 0.69 ± 0.01b 0.24 ± 0.01c 变异来源
Source of variation品种 Variety (V) ns *** *** *** ns 15% PEG (P) ns *** *** *** *** V × P ns ns * ns ns Fo:初始荧光;Fm:最大荧光; Fv/ Fo:PSⅡ潜在效率;Fv/ Fm:最大光化学效率;qP:光化学淬灭系数。
Fo: minimal fluorescence; Fm: maximal fluorescence; Fv/ Fo: potential efficiency of PSⅡ; Fv/ Fm: maximal photochemical efficiency; qP: photochemical quenching coefficient.2.5 干旱胁迫对甘草幼苗整株根系水力学导度的影响
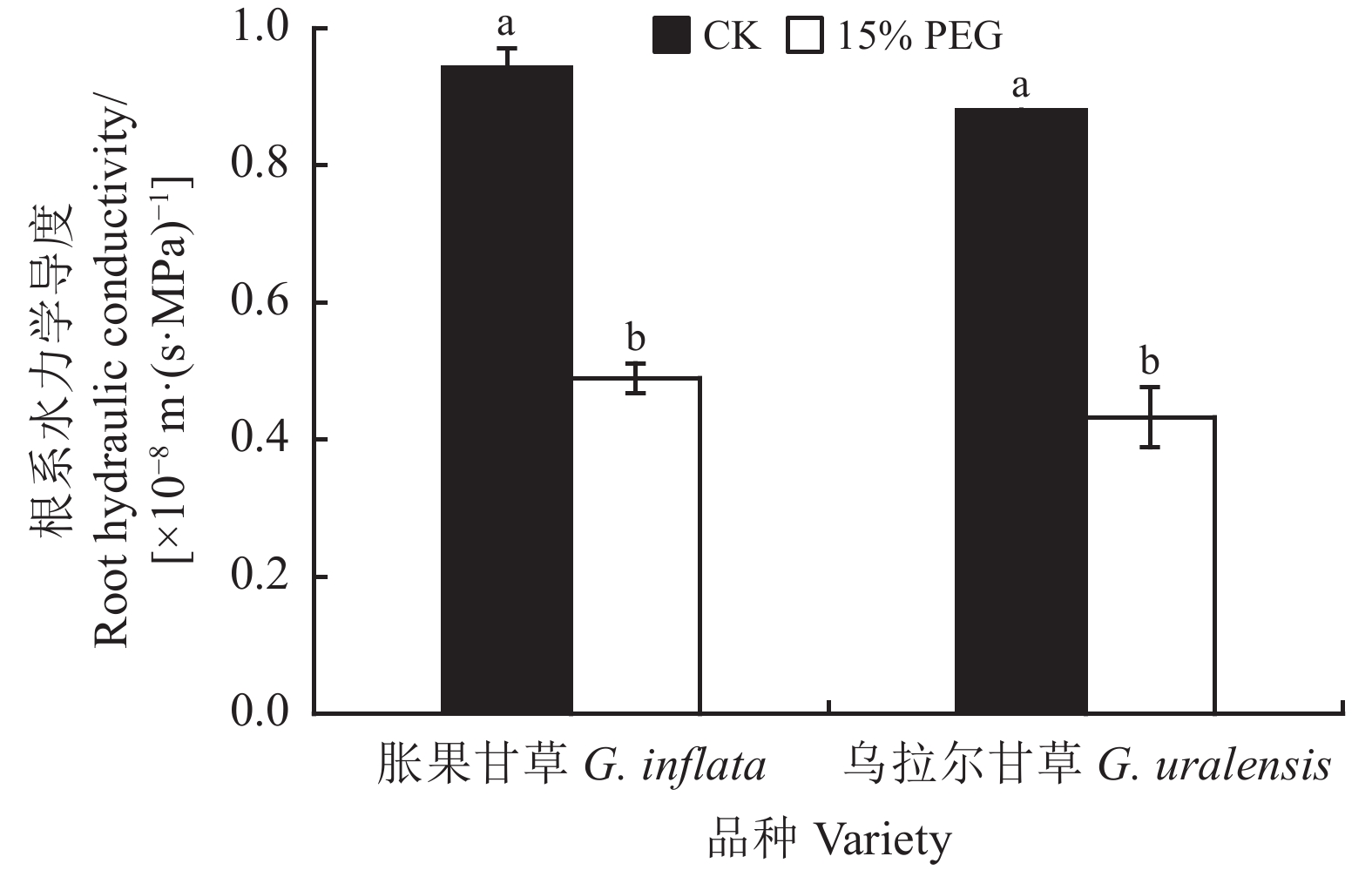
正常水分条件下,胀果甘草和乌拉尔甘草幼苗的整株根系水力学导度分别为0.94 × 10−8 和0.88 × 10−8 m·(s·MPa)−1 (图3)。干旱胁迫下胀果甘草和乌拉尔甘草幼苗的根系水力学导度分别为0.48 × 10−8、0.43 × 10−8 m·(s·MPa)−1,显著降低48.94%和51.14% (P < 0.05);可见与正常水分条件相比,干旱胁迫均显著降低了两个甘草品种的根系水力学导度(P < 0.05),但胀果甘草的根系水力学导度仍高于乌拉尔甘草,且胀果甘草幼苗根系水力学导度下降幅度小于乌拉尔甘草。
3. 讨论
3.1 干旱胁迫对甘草幼苗光合特性和生长的影响
干旱是限制植物生长的重要因素,本研究中干旱胁迫降低了甘草的Pn,且与Tr、Gs呈同步变化,限制其地上部光合作用,造成植物整体干物质累积受限[35-36],这与前人对白杨(Populus przewalskii) [36]、老芒麦(Elymus sibiricus) [37]等的研究结果一致,而WUE在干旱胁迫下增加的原因可能是由于水分蒸腾散失对气孔的依赖大于光合对气孔的依赖,因此Pn降幅小于水分消耗的比率,使甘草WUE提高。干旱胁迫导致光合作用降低的原因分为气孔因素和非气孔因素[38],研究认为干旱胁迫下植物的第一反应是关闭气孔,减少蒸腾作用以维持叶片水分平衡,这也是轻度及中度干旱胁迫早期光合作用下降的主要限制因素[39],随着干旱程度加剧或干旱时间的延长,光合作用的限制因子会发生改变,Ci升高,非气孔因素成为限制光合作用的主要因素,在沙棘(Hippophae rhamnoides) [40]、金银花(Lonicera japonica) [41]等植物中均发现这种规律。本研究中,与CK相比,15% PEG胁迫7 d后甘草叶片Ci下降,与项雯慧等[38]的研究结果一致,说明Pn降低是由气孔因素引起的,气孔作为叶片内部与外界进行气体和水分交流的通道,适度关闭气孔减少蒸腾损失有助于植物在干旱胁迫下保持水分,这是植物对干旱胁迫的主动适应性反应。干旱胁迫下,胀果甘草可以保持较强的光合作用并降低蒸腾速率,有助于更多光合产物的形成和更强的蒸腾拉力促进根部吸收更多的水分。这可能是由于胀果甘草幼苗叶片气孔可以进行规律性的开合振荡[42],减少气孔阻力和水分蒸发损失的同时保持较高的水分利用效率,增强胀果甘草的抗逆性。Fv/Fm表示PSⅡ反应中心利用光能的能力,被用来监测植株在逆境中的受胁迫程度[43],本研究中甘草幼苗Fv/Fm、光化学淬灭系数qP在胁迫下均降低,这与Gao等[44]的研究结果一致,说明此时PSⅡ反应中心受到了抑制或损伤,PSⅡ天线色素吸收的光能用于光反应的份额减少,PSⅡ反应中心的光能过剩[45],致使其光合效率降低[46]。在干旱胁迫下,胀果甘草的Fv/Fm和qP相对降低幅度较小,说明干旱胁迫对其叶片的损伤程度较轻,在PSⅡ部分关闭时具有较高的光化学效率[47],因此胀果甘草具有较高光合作用优势。
3.2 干旱胁迫对甘草幼苗根系吸水的影响
在植物生长过程中,根系水分吸收和叶片水分流失之间存在动态平衡[48]。本研究干旱胁迫下甘草幼苗叶片相对含水量和叶水势均降低,其中胀果甘草的降幅小于乌拉尔甘草,与Zhang等[49]的研究结果一致,这是由于植物根系吸收水分向茎干以及叶片运移受到抑制[50]。前人研究发现,抗旱性强的小麦品种具有较强的根系水力学导度[19],本研究中两种甘草干旱胁迫下整株根系水力学导度均显著降低,且胀果甘草下降幅度小于乌拉尔甘草并保持较高值。蒸腾拉力是植物根系吸水的主要驱动力,蒸腾作用的强弱直接影响植物的蒸腾拉力,而干旱胁迫下胀果甘草具有更高的蒸腾速率,且胀果甘草叶片气孔有规律的开合可以减弱气孔阻力,有效提升根系的蒸腾拉力,因此与乌拉尔甘草相比胀果甘草具有更强的水分吸收能力,表明胀果甘草具有较强的抗旱性,与王常贵[51]对甘草属植物解剖学研究的结论一致。
干旱胁迫下甘草幼苗根系增粗受到抑制但总根长显著增加,这是因为植物响应干旱胁迫的策略为先适应后迫害,较长的根系有利于拓宽水分吸收面积,提高水分利用率[52]。普遍认为根系越庞大的作物获得的水分和养分越多,Hill等[53]认为抗旱性强的马铃薯(Solanum tuberosum)具有更深的生根系统或高根密度。但也有研究表明在一定范围内根系的差异并不一定引起地上部的差异[54]。干旱胁迫对植物生长的抑制作用具有组织特异性,研究发现干旱胁迫对甘草冠层生长抑制程度大于根系,根冠比增加,促进干物质向根系分配[55-56]。植物的根冠关系受遗传和环境因素耦合影响,根冠生长比例协调时具有较高的资源利用效率[57]。马富举等[58]认为干旱条件下耐旱型品种可以维持较高的根系生长量是维持根系水分吸收能力的重要基础。而本研究中胀果甘草的根长、根表面积和根平均直径均小于乌拉尔甘草,但其具有更强的抗旱能力,这是由于超过50%的光合产物通过根呼吸损失,较小的根系具有更高的根系效率,通过更强的根系吸水能力提高了作物水分利用效率[59]。
4. 结论
干旱胁迫下,甘草幼苗光合作用被抑制,PSⅡ部分失活,整株根系水力学导度降低,但甘草幼苗可通过促进根系伸长拓宽水分吸收面积、降低叶片蒸腾作用提高水分利用效率,以增强抗旱性。两个甘草品种中,胀果甘草具有较高光合作用优势,同时其相比乌拉尔甘草根系较小,具有更高的根系效率可维持较高的根系吸水能力。因此,品种间胀果甘草幼苗抗旱能力较强。
参考文献
[1] LESK C, ROWHANI P, RAMANKUTTY N. Influence of extreme weather disasters on global crop production. Nature, 2016, 529: 84-87. doi: 10.1038/nature16467
[2] SALLAM A, ALQUDAH A M, DAWOOD M F A, BAENZIGER P S, BORNER A. Drought stress tolerance in wheat and barley advances in physiology, breeding and genetics Research. International Journal of Molecular Sciences, 2019, 20(13): 3137. doi: 10.3390/ijms20133137
[3] WILLCOCK S, COOPER G S, ADDY J, DEARING J A. Earlier collapse of anthropocene ecosystems driven by multiple faster and noisier drivers. Nature Sustainability, 2023, 6: 1331-1342.
[4] JIN Y, CHANG J, SHI Y, ZHANG Q, XIA C. Effects of drought stress on growth, photosynthesis and ultrstructure of mesophyll cell of ulmus szechuanica fang seedlings. Bangladesh Journal of Botany, 2022, 51(3): 461-468. doi: 10.3329/bjb.v51i3.61992
[5] ZAHRA N, HAFEEZ M B, KAUSAR A, Al Z M, ASEKOVA S, SIDDIQUE K H, FAROOQ M. Plant photosynthetic responses under drought stress: Effects and management. Journal of Agronomy and Crop Science, 2023, 209(5): 651-672.
[6] 赵霖玉, 李秧秧. 遮阴和干旱对白桦幼苗光诱导的气孔动力学影响. 应用生态学报, 2022, 33(9): 2331-2338. doi: 10.13287/j.1001-9332.202209.004 ZHAO L Y, LI Y Y. Effects of shading and drought on light-induced stomatal dynamics in betula platyphylla seedlings. Chinese Journal of Applied Ecology, 2022, 33(9): 2331-2338. doi: 10.13287/j.1001-9332.202209.004
[7] 杨小龙, 须晖, 李天来, 王蕊. 外源褪黑素对干旱胁迫下番茄叶片光合作用的影响. 中国农业科学, 2017, 50(16): 3186-3195. doi: 10.3864/j.issn.0578-1752.2017.16.013 YANG X L, XU H, LI T L, WANG R. Effects of melatonin on photosynthesis of tomato leaves under drought stress. Scientia Agricultura Sinica, 2017, 50(16): 3186-3195. doi: 10.3864/j.issn.0578-1752.2017.16.013
[8] BANO H, ATHAR H U R, ZAFAR Z U, KALAJI H M, ASHRAF M. Linking changes in chlorophyⅡ a fluorescence with drought stress susceptibility in mung bean ( Vigna radiata L. Wilczek). Physiol Plantarum, 2021, 172(2): 1244-1254. doi: 10.1111/ppl.13327
[9] 娄成后. 植物水分平衡中根冠间的信号传递与整体行动. 植物学通报, 2000, 17(5): 475-477. LOU C H. Signal transmission and overall action between root and shoot in plant water balance. Chinese Bulletin of Botany, 2000, 17(5): 475-477.
[10] 王卫锋. 植物整体水分平衡的生理生态调控机制研究. 杨凌: 中国科学院大学(教育部水土保持与生态环境研究中心)博士学位论文, 2013. WANG W F. The ecophysiological mechanisms of maintaining whole-plant water balance. PhD Thesis. Yangling: University of Chinese Academy of Science (Research Center of Soil and Water Conservation and Ecological Environment), 2013.
[11] 康绍忠. 土壤-植物-大气连续体水分传输动力学及其应用. 力学与实践, 1993, 15(1): 11-19. KANG S Z. Dynamics of water transport in soil-plant-atmosphere continuum and its application. Mechanics in Engineering, 1993, 15(1): 11-19.
[12] 苗青霞, 方燕, 陈应龙. 小麦根系特征对干旱胁迫的响应. 植物学报, 2019, 54(5): 652-661. doi: 10.11983/CBB19089 MIAO Q X, FANG Y, CHEN Y L. Response of wheat root characteristics to drought stress. Chinese Bulletin of Botany, 2019, 54(5): 652-661. doi: 10.11983/CBB19089
[13] BASU S, RAMEGOWDA V, KUMAR A, PEREIRA A. Plant adaptation to drought stress. F1000Research, 2016, 5: 1554.
[14] MANOJ K, RAJU S, SATOSHI O, YUSAKU U, MICHAEL G S, SATEESH K. Drought response in wheat: Key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Frontiers in Chemistry, 2017(5): 106. doi: 10.3389/fchem.2017.00106
[15] KOEVOETS I T, HENKV J, ELZENGA J T M, TESTERINK C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Frontiers in Plant Science, 2016, 7: 1335.
[16] FROMM H. Root plasticity in the pursuit of water. Plants, 2019, 8(7): 236. doi: 10.3390/plants8070236
[17] SERGIO J, JIHENE D, DIEGO G, MORET D, IRIGOYEN J J, MORENO M A, GOGORCENA Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiology, 2013(10): 1061-1075.
[18] 李文娆, 李小利, 张岁岐, 山仑. 水分亏缺下紫花苜蓿和高粱根系水力学导度与水分利用效率的关系. 生态学报, 2011, 31(5): 1323-1333. LI W R, LI X L, ZHANG S Q, SHAN L. The root system hydraulic conductivity and water use efficiency of alfalfa and sorghum under water deficit. Acta Ecologica Sinica, 2011, 31(5): 1323-1333.
[19] 王荣荣, 王海琪, 蒋桂英, 尹豪杰, 谢冰莹, 张婷. 2个不同抗旱性小麦品种耗水特征及根系生理特性对开花期干旱的响应. 水土保持学报, 2022, 36(4): 253-264. doi: 10.13870/j.cnki.stbcxb.2022.04.032 WANG R R, WANG H Q, JIANG G Y, YIN H J, XIE B Y, ZHANG T. Responses of water consumption and root physiological characteristics of two different drought-tolerant wheat varieties to anthesis drought. Journal of Soil and Water Conservation, 2022, 36(4): 253-264. doi: 10.13870/j.cnki.stbcxb.2022.04.032
[20] 忽雪琦, 李东阳, 严加坤, 张岁岐. 干旱胁迫下外源茉莉酸甲酯对玉米幼苗根系吸水的影响. 植物生理学报, 2018, 54(5): 991-998. doi: 10.13592/j.cnki.ppj.2018.0136 HU X Q, LI D Y, YAN J K, ZHANG S Q. Impact of exogenous methyl jasmonate on water absorption of maize roots under drought stress. Plant Physiology Journal, 2018, 54(5): 991-998. doi: 10.13592/j.cnki.ppj.2018.0136
[21] 张海旺, 钟启文, 娄祥云, 杨世鹏, 谭龙, 孙雪梅, 王丽慧. 干旱胁迫对不同基因型菊芋生理特性的影响. 植物生理学报, 2022, 58(12): 2312-2320. doi: 10.13592/j.cnki.ppj.100184 ZHANG H W, ZHONG Q W, LOU X Y, YANG S P, TAN L, SUN X M, WANG L H. Effects of drought stress on physiological characteristics of different genotypes of Helianthus tuberosus. Plant Physiology Journal, 2022, 58(12): 2312-2320. doi: 10.13592/j.cnki.ppj.100184
[22] 于国红, 刘朋程, 郝洪波, 崔海英, 郭安强, 李明哲. 不同基因型谷子对干旱胁迫的调控机制. 植物营养与肥料学报, 2022, 28(1): 157-167. doi: 10.11674/zwyf.2021134 YU G H, LIU P C, HAO H B, CUI H Y, GUO A Q, LI M Z. Regulation mechanism of drought resistance in different genotypes of foxtail millet. Journal of Plant Nutrition and Fertilizers, 2022, 28(1): 157-167. doi: 10.11674/zwyf.2021134
[23] 曾令霜, 李培英, 孙宗玖, 孙晓梵. 两类新疆狗牙根抗旱基因型抗氧化酶保护系统及其基因表达差异分析. 草业学报, 2022, 31(7): 122-132. doi: 10.11686/cyxb2021178 ZENG L S, LI P Y, SUN Z J, SUN X F. Analysis of antioxidant enzyme protection systems and gene expression differences in two Xinjiang bermudagrass genotypes with contrasting drought resistance. Acta Prataculturae Sinica, 2022, 31(7): 122-132. doi: 10.11686/cyxb2021178
[24] 赵小强, 陆晏天, 白明兴, 徐明霞, 彭云玲, 丁永福, 庄泽龙, 陈奋奇, 张大志. 不同株型玉米基因型对干旱胁迫的响应分析. 草业学报, 2020, 29(2): 149-162. ZHAO X Q, LU Y T, BAI X M, XU M X, PENG Y L, DING Y F, ZHUANG Z L, CHEN F Q, ZHANG D Z. Responses of maize genotypes with different plant architecture to drought stress. Acta Prataculturae Sinica, 2020, 29(2): 149-162.
[25] SHUAI J, LI Z, WEI S, WANG Y, MIN Y. Bioactive constituents of Glycyrrhiza uralensis (Licorice): Discovery of the effective components of a traditional herbal medicine. Journal of Natural Products, 2017, 79(2): 281-292.
[26] 盛玉章. 水盐胁迫及施肥对甘草和秦艽品质的影响. 杨凌: 西北农林科技大学硕士学位论文, 2022. SHENG Y Z. Effects of water and salt stress and fertilization on the quality of Glycyrrhiza uralensis Fisch. and G. macrophylla Pall. Master Thesis. Yangling: Northwest Agriculture and Forestry University, 2022.
[27] 耿广琴, 谢晓蓉. 旱盐双重胁迫对乌拉尔甘草幼苗生理生化特性的影响. 草业科学, 2018, 35(9): 2166-2173. doi: 10.11829/j.issn.1001-0629.2017-0464 GENG G Q, XIE X R. Effect of drought and salt stress on the physiological and biochemical characteristics of Glycyrrhiza uralensis. Pratacultural Science, 2018, 35(9): 2166-2173. doi: 10.11829/j.issn.1001-0629.2017-0464
[28] 李宗谕, 刘福顺, 刘秀岩, 杨世海. 盐碱胁迫和干旱胁迫对胀果甘草种子萌发及幼苗生长和内源激素影响. 时珍国医国药, 2020, 31(6): 1464-1467. doi: 10.3969/j.issn.1008-0805.2020.06.062 LI Z Y, LIU F S, LIU X Y, YANG S H. Effects of saline-alkali stress and drought stress on seed germination, seedling growth and endogenous hormones of Glycyrrhiza inflata Batalin. Lishizhen Medicine and Materia Medica Research, 2020, 31(6): 1464-1467. doi: 10.3969/j.issn.1008-0805.2020.06.062
[29] 张东, 刘艳, 张晗, 王洋, 张子健, 刘美岑. 甘草叶片渗透调节物质及蔗糖代谢相关酶对干旱胁迫的响应特性. 西北植物学报, 2020, 40(5): 819-827. ZHANG D, LIU Y, ZHANG H, WANG Y, ZHANG Z J, LIU M C. Response of osmotic regulator and sucrose metabolization-related enzymes to drought stress in Glycyrrhiza uralensis. Acta Botanica Boreali-Occidentalia Sinica, 2020, 40(5): 819-827.
[30] 安钰, 张清云, 李生兵, 刘华. 干旱胁迫及复水对甘草叶片抗氧化酶活性和光合特性的影响. 宁夏农林科技, 2021, 62(7): 1-5. doi: 10.3969/j.issn.1002-204x.2021.07.001 AN Y, ZHANG Q Y, LI S B, LIU H. Effect of drought stress and rehydration on antioxidant enzyme activity and photosynthetic characteristic of Glycyrrhiza uralensis. Journal of Ningxia Agriculture and Forestry Science and Technology, 2021, 62(7): 1-5. doi: 10.3969/j.issn.1002-204x.2021.07.001
[31] 关思静, 王楠, 徐蓉蓉, 葛甜甜, 高静, 彭亮, 张岗, 陈莹. 甘草幼苗响应干旱胁迫的光合、抗氧化特性及转录组分析. 草业科学, 2021, 38(11): 2176-2190. GUAN S J, WANG N, XU R R, GE T T, GAO J, PENG L, ZHANG G, CHEN Y. Photosynthetic, antioxidant enzyme activity, and transcriptome sequencing analyses of Glycyrrhiza uralensis seedings in response to drought stress. Pratacultural Science, 2021, 38(11): 2176-2190.
[32] 刘长乐, 寇佩雯, 许祎珂, 张永生, 高静, 贺壮壮, 李帅帅, 黄文静. 逆境胁迫和植物生长调节剂对甘草种子萌发的影响. 中国野生植物资源, 2022, 41(2): 14-20. LIU C L, KOU P W, XU Y K, ZHANG Y S, GAO J, HE Z Z, LI S S, HUANG W J. Effect of adversity stress and plant growth Regulator on seed germination of Glycyrrhiza uralensis Fish. Chinese Wild Plant Resources, 2022, 41(2): 14-20.
[33] YAN J K, ZHANG N N, WANG N, LI Y P, ZHANG S Q, WANG S W. Variations in adaptation strategies of wheat cultivar replacements under short-term osmotic stress. Pakistan Journal of Botany, 2016, 48(3): 917-924.
[34] ZHAO C X, DENG X P, SHAN L, STEUDLE E, ZHANG S Q, YE Q. Changes in root hydraulic conductivity during wheat evolution. Journal of Integrative Plant Biology, 2005, 47(3): 302-310. doi: 10.1111/j.1744-7909.2005.00043.x
[35] 朱铁霞, 高阳, 高凯, 李志华. 干旱胁迫下菊芋各器官生物量及物质分配规律. 生态学报, 2019, 39(21): 8021-8026. ZHU T X, GAO Y, GAO K, LI Z H. Organ biomass and resource allocation in response to drought stress in jerusalem artichoke. Acta Ecologica Sinica, 2019, 39(21): 8021-8026.
[36] YIN C Y, BERNINGER F, LI C Y. Photosynthetic responses of Populus przewalskii subjected to drought stress. Photosynthetica, 2006, 44(1): 62-68.
[37] 王传旗. 水分亏缺及复水下老芒麦生理生态特性及耐旱性研究. 西宁: 青海大学博士学位论文, 2022. WANG C Q. Studies on physiological ecology characteristics and drought tolerance of Elymus sibiricus under water deficit and rewatering. PhD Thesis. Xining: Qinghai University, 2022.
[38] 项雯慧, 张林平, 王洋, 刘艳. 不同浓度PEG胁迫对甘草幼苗光合特性的影响. 内蒙古农业大学学报(自然科学版), 2020, 41(1): 19-24. doi: 10.16853/j.cnki.1009-3575.2020.01.004 XIANG H W, ZHANG L P, WANG Y, LIU Y. Effects of different concentrations of PEG-6000 stress on photosynthetic characteristics of Glycyrrhiza young seedlings. Journal of Inner Mongolia Agricultural University (Natural Science Edition), 2020, 41(1): 19-24. doi: 10.16853/j.cnki.1009-3575.2020.01.004
[39] UTKHAO W, YINGJAJAWAL S. Changes in leaf gas exchange and biomass of Eucalyptus camaldulesis in response to increasing drought stress induced by polyethylene glycol. Trees, 2015, 29(5): 1581-1592. doi: 10.1007/s00468-015-1240-1
[40] 刘歌畅, 岳丽华, 刘春海, 赵鹏智, 邓子易, 李玉灵. 干旱胁迫对俄罗斯大果沙棘幼苗光合荧光参数的影响. 北方园艺, 2022, 517(22): 16-23. LIU G C, YUE L H, LIU C H, ZHAO P Z, DENG Z Y, LI Y L. Effects of drought stress on photosynthetic fluorescence parameters of Hippophae rhamnoides Linn. Seedings. Northern Horticulture, 2022, 517(22): 16-23.
[41] ZHAO J, LANG Y, ZHANG S Y, ZHAO Q K, ZHANG C Y, XIA J B. Photosynthetic characteristics and chlorophyⅡ a fluorescence transient in Lonicera japonica under drought stress. Acta Physiologiae Plantarum, 2019, 41(7): 124.
[42] 王根轩, 廖建雄, 吴冬秀. 荒漠条件下甘草气孔振荡的水被动证据. 植物学报, 2001, 43(1): 41-45. WANG G X, LIAO J X, WU D X. Evidence of hydropassive movement in stomatal oscillations of Glycyrrhiza inflata under desert conditions. Journal of Integrative Plant Biology, 2001, 43(1): 41-45.
[43] 刘文瑜, 杨发荣, 黄杰, 魏玉明, 李健荣. 干旱胁迫对藜麦幼苗生长和叶绿素荧光特性的影响. 干旱地区农业研究, 2019, 37(4): 171-177. LIU W Y, YANG F R, HUANG J, WEI Y M, LI J R. Effect of drought stress on chlorophyⅡ fluorescence properties and seedling growth of quinoa seedlings. Agriculture Research in the Arid Areas, 2019, 37(4): 171-177.
[44] GAO H, BAI N, ZANG Y, ZHANG Y J, WANG L, WANG E J, TIAN Y Y, GUO Y Y, YAN F, LI Y H, ZHANG H. Drought stress alters gas exchange, chlorophyⅡ fluorescence, and antioxidant enzyme activities in Glycyrrhiza uralensis in the Hexi Corridor, China. Russian Journal of Plant Physiology, 2022, 69(6): 123.
[45] GUO Y Y, YU H Y, KONG D S, FAN F, ZHANG Y J. Effects of drought stress on growth and chlorophyⅡ fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica, 2016, 54(4): 524-531. doi: 10.1007/s11099-016-0206-x
[46] WANG J, LU W, TONG Y, YANG Q. Leaf morphology, photosynthetic performance, chlorophyⅡ fluorescence, stomatal development of lettuce ( Lactucasativa L.) exposed to different ratios of red light to blue light. Frontiers in Plant Science, 2016, 7: 250.
[47] 庞进平, 王永生. 油菜幼苗光合及叶绿素荧光参数对干旱胁迫的响应及其抗旱性分析. 西北植物学报, 2023, 43(2): 276-284. doi: 10.7606/j.issn.1000-4025.2023.02.0276 PANG J P, WANG Y S. Photosynthetic and chlorophyⅡ fluorescence responses of rape seedlings to drought stress and its drought resistance evaluation. Acta Botanica Boreali-Occidentalia Sinica, 2023, 43(2): 276-284. doi: 10.7606/j.issn.1000-4025.2023.02.0276
[48] YAN M, ZHANG C, LI H, ZHANG L, REN Y, CHEN Y, CAI H, ZHANG S. Root pruning improves maize water-use efficiency by root water absorption. Frontiers in Plant Science, 2023, 13: 1023088. doi: 10.3389/fpls.2022.1023088
[49] ZHANG W J, XIE Z C, ZHANG X J, LANG D Y, ZHANG X H. Growth-promoting bacteria alleviates drought stress of G. uralensis through improving photosynthesis characteristics and water status. Journal of Plant Interactions, 2019, 14(1): 580-589. doi: 10.1080/17429145.2019.1680752
[50] 蒋涛, 郑文革, 余新晓, 贾国栋, 孜尔蝶·巴合提, 张欢, 王渝淞, 王玥璞, 丁兵兵. 北京山区干旱胁迫下侧柏叶片水分吸收策略. 生态学报, 2022, 42(4): 1429-1440. JIANG T, ZHENG W G, YU X X, JIA G D, Zierdie·Baheti, ZHANG H, WANG Y S, WANG Y S, WANG Y P, DING B B. Leaf water absorption of Platycladus orientalis under drought stress in Beijing. Acta Ecologica Sinica, 2022, 42(4): 1429-1440.
[51] 王常贵, 管绍淳, 杨戈. 新疆甘草属植物的解剖学研究. 干旱区研究, 1984(2): 44-48. doi: 10.13866/j.azr.1984.02.009 WANG C G, GUAN S C, YANG G. Anatomical study on Glycyrrhiza L. plants in Xinjiang. Arid Zone Research, 1984(2): 44-48. doi: 10.13866/j.azr.1984.02.009
[52] 李文娆, 张岁岐, 丁圣彦, 山仑. 干旱胁迫下紫花苜蓿根系形态变化及与水分利用的关系. 生态学报, 2010(19): 5140-5150. LI W R, ZHANG S Q, DING S Y, SHAN L. Root morphological variation and water use in alfalfa under drought stress. Acta Ecologica Sinica, 2010(19): 5140-5150.
[53] HILL D, NELSON D, HAMMOND J, BELL L. Morphophysiology of potato ( Solanum tuberosum) in response to drought stress: Paving the way forward. Frontiers in Plant Science, 2021, 11: 597554. doi: 10.3389/fpls.2020.597554
[54] 苗果园, 高志强, 张云亭, 尹钧, 张爱良. 水肥对小麦根系整体影响及其与地上部相关的研究. 作物学报, 2002, 28(4): 445-450. MIAO G Y, GAO Z Q, ZHANG Y T, YIN J, ZHANG A L. Effect of water and fertilizer to root system and its correlation with tops in wheat. Acta Agronomica Sinica, 2002, 28(4): 445-450.
[55] XIA J B, ZHANG S Y, LI T, LIU R H, GUANG C. Effect of continuous cropping generations on each component biomass of poplar seedlings during different growth periods. The Scientific World Journal, 2014(2): 618421.
[56] 陈明涛, 赵忠. 干旱对4种苗木根系特征及各部分物质分配的影响. 北京林业大学学报, 2011, 33(1): 16-22. doi: 10.13332/j.1000-1522.2011.01.016 CHEN M T, ZHAO Z. Effects of drought on root characteristics and mass allocation in each part of seedlings of four tree species. Journal of Beijing Forestry University, 2011, 33(1): 16-22. doi: 10.13332/j.1000-1522.2011.01.016
[57] 张岁岐. 根冠关系对作物水分利用的调控. 杨凌: 西北农林科技大学博士学位论文, 2001. ZHANG S Q. Regulation of root-shoot relationship on crop water use. PhD Thesis. Yangling: Northwest Agriculture and Forestry University, 2001.
[58] 马富举, 李丹丹, 蔡剑, 姜东, 曹卫星, 戴廷波. 干旱胁迫对小麦幼苗根系生长和叶片光合作用的影响. 应用生态学报, 2012, 23(3): 724-730. doi: 10.13287/j.1001-9332.2012.0097 MA F J, LI D D, CAI J, JIANG D, CAO W X, DAI T B. Responses of wheat seedings root growth and leaf photosynthesis to drought stress. Chinese Journal of Applied Ecology, 2012, 23(3): 724-730. doi: 10.13287/j.1001-9332.2012.0097
[59] YAN M, ZHANG L, REN Y, ZHANG T, ZHANG S, LI H, CHEN Y, ZHANG S. The higher water absorption capacity of small root system improved the yield and water use efficiency of Maize. Plants, 2022, 11(17): 112300.
-
图 1 干旱胁迫下甘草叶片相对含水量和叶水势
不同小写字母表示不同处理间差异显著(P < 0.05);下图同。
Figure 1. Influence of leaf relative water content and water potential of Glycyrrhiza inflata and G. uralensis on drought stress
Different lowercase letters indicate significant differences between different treatments at the 0.05 level. This is applicable for the following figures as well.
表 1 干旱胁迫对甘草生物量和根冠比的影响
Table 1 Effects of drought stress on biomass and root-shoot ratio of Glycyrrhiza inflata and G. uralensis
品种
Variety处理
Treatment地上部鲜重
Shoot fresh
weight/g地上部干重
Shoot dry
weight/g根鲜重
Root fresh
weight/g根干重
Root dry
weight/g总干重
Total dry
weight/g根冠比
Root-shoot
ratio/%胀果甘草
G. inflataCK 2.17 ± 0.21b 0.41 ± 0.14b 0.79 ± 0.10bc 0.06 ± 0.01bc 0.47 ± 0.14b 17.17 ± 5.67ab 15% PEG 0.69 ± 0.06c 0.18 ± 0.03c 0.49 ± 0.12c 0.05 ± 0.00b 0.23 ± 0.03c 26.59 ± 4.83a 乌拉尔甘草
G. uralensisCK 6.04 ± 0.47a 1.12 ± 0.09a 1.96 ± 0.37a 0.14 ± 0.03a 1.27 ± 0.11a 12.61 ± 1.79b 15% PEG 1.06 ± 0.08c 0.42 ± 0.05b 1.16 ± 0.29b 0.10 ± 0.03ab 0.52 ± 0.08b 24.43 ± 4.07a 变异来源
Source of variation品种 Variety (V) *** *** *** *** *** ns 15% PEG (P) *** *** * ns *** ** V × P *** ** ns ns ** ns 同列不同小写字母表示差异显著(P < 0.05)。CK:对照;15% PEG:Hoagland全营养液配制15% PEG-6000溶液模拟干旱胁迫。*、**、***分别表示P < 0.05、0.01、0.001;ns:不显著,P ≥ 0.05。下同。
Different lowercase letters in the same column represent significant difference at the 0.05 level. CK: control; 15% PEG: 15% PEG-6000 solution prepared with Hoagland nutrient solution was used to simulate drought stress. *, **, *** indicate P < 0.05, 0.01, 0.001, respectively; ns: not significant, P ≥ 0.05. This is applicable for the following figures and tables as well.表 2 干旱胁迫对两种甘草根系构型的影响
Table 2 Effects of drought stress on total root length, total root surface area, root volume, and mean root diameter of Glycyrrhiza inflata and G. uralensis
品种
Variety处理
Treatment总根长
Total root length/cm总根表面积
Total root surface area/cm2根体积
Root volume/cm3平均直径
Mean root diameter/mm胀果甘草
G. inflataCK 352.94 ± 8.13d 83.34 ± 1.42b 1.13 ± 0.02a 0.51 ± 0.06b 15% PEG 441.96 ± 11.39c 65.56 ± 8.51c 1.03 ± 0.34a 0.43 ± 0.01c 乌拉尔甘草
G. uralensisCK 523.20 ± 49.21b 118.34 ± 10.69a 1.35 ± 0.54a 0.62 ± 0.03a 15% PEG 627.69 ± 15.01a 89.90 ± 3.32b 1.24 ± 0.02a 0.52 ± 0.01b 变异来源
Source of variation品种 Variety (V) *** *** ns ** 15% PEG (P) *** ** ns ** V × P ns ns ns ns 表 3 干旱胁迫下甘草叶绿素荧光参数
Table 3 Effects of chlorophyⅡ fluorescence parameters of Glycyrrhiza inflata and G. uralensis on drought stress
品种
Variety处理
TreatmentFo Fm Fv/Fo Fv/Fm qP 胀果甘草
G. inflataCK 0.089 ± 0.004ab 0.26 ± 0.01c 1.96 ± 0.01b 0.66 ± 0.01b 0.34 ± 0.04a 15% PEG 0.090 ± 0.002ab 0.23 ± 0.02c 1.53 ± 0.17c 0.60 ± 0.03c 0.28 ± 0.01bc 乌拉尔甘草
G. uralensisCK 0.088 ± 0.004b 0.38 ± 0.02a 3.39 ± 0.32a 0.77 ± 0.02a 0.33 ± 0.02ab 15% PEG 0.096 ± 0.002a 0.31 ± 0.01b 2.20 ± 0.03b 0.69 ± 0.01b 0.24 ± 0.01c 变异来源
Source of variation品种 Variety (V) ns *** *** *** ns 15% PEG (P) ns *** *** *** *** V × P ns ns * ns ns Fo:初始荧光;Fm:最大荧光; Fv/ Fo:PSⅡ潜在效率;Fv/ Fm:最大光化学效率;qP:光化学淬灭系数。
Fo: minimal fluorescence; Fm: maximal fluorescence; Fv/ Fo: potential efficiency of PSⅡ; Fv/ Fm: maximal photochemical efficiency; qP: photochemical quenching coefficient. -
[1] LESK C, ROWHANI P, RAMANKUTTY N. Influence of extreme weather disasters on global crop production. Nature, 2016, 529: 84-87. doi: 10.1038/nature16467
[2] SALLAM A, ALQUDAH A M, DAWOOD M F A, BAENZIGER P S, BORNER A. Drought stress tolerance in wheat and barley advances in physiology, breeding and genetics Research. International Journal of Molecular Sciences, 2019, 20(13): 3137. doi: 10.3390/ijms20133137
[3] WILLCOCK S, COOPER G S, ADDY J, DEARING J A. Earlier collapse of anthropocene ecosystems driven by multiple faster and noisier drivers. Nature Sustainability, 2023, 6: 1331-1342.
[4] JIN Y, CHANG J, SHI Y, ZHANG Q, XIA C. Effects of drought stress on growth, photosynthesis and ultrstructure of mesophyll cell of ulmus szechuanica fang seedlings. Bangladesh Journal of Botany, 2022, 51(3): 461-468. doi: 10.3329/bjb.v51i3.61992
[5] ZAHRA N, HAFEEZ M B, KAUSAR A, Al Z M, ASEKOVA S, SIDDIQUE K H, FAROOQ M. Plant photosynthetic responses under drought stress: Effects and management. Journal of Agronomy and Crop Science, 2023, 209(5): 651-672.
[6] 赵霖玉, 李秧秧. 遮阴和干旱对白桦幼苗光诱导的气孔动力学影响. 应用生态学报, 2022, 33(9): 2331-2338. doi: 10.13287/j.1001-9332.202209.004 ZHAO L Y, LI Y Y. Effects of shading and drought on light-induced stomatal dynamics in betula platyphylla seedlings. Chinese Journal of Applied Ecology, 2022, 33(9): 2331-2338. doi: 10.13287/j.1001-9332.202209.004
[7] 杨小龙, 须晖, 李天来, 王蕊. 外源褪黑素对干旱胁迫下番茄叶片光合作用的影响. 中国农业科学, 2017, 50(16): 3186-3195. doi: 10.3864/j.issn.0578-1752.2017.16.013 YANG X L, XU H, LI T L, WANG R. Effects of melatonin on photosynthesis of tomato leaves under drought stress. Scientia Agricultura Sinica, 2017, 50(16): 3186-3195. doi: 10.3864/j.issn.0578-1752.2017.16.013
[8] BANO H, ATHAR H U R, ZAFAR Z U, KALAJI H M, ASHRAF M. Linking changes in chlorophyⅡ a fluorescence with drought stress susceptibility in mung bean ( Vigna radiata L. Wilczek). Physiol Plantarum, 2021, 172(2): 1244-1254. doi: 10.1111/ppl.13327
[9] 娄成后. 植物水分平衡中根冠间的信号传递与整体行动. 植物学通报, 2000, 17(5): 475-477. LOU C H. Signal transmission and overall action between root and shoot in plant water balance. Chinese Bulletin of Botany, 2000, 17(5): 475-477.
[10] 王卫锋. 植物整体水分平衡的生理生态调控机制研究. 杨凌: 中国科学院大学(教育部水土保持与生态环境研究中心)博士学位论文, 2013. WANG W F. The ecophysiological mechanisms of maintaining whole-plant water balance. PhD Thesis. Yangling: University of Chinese Academy of Science (Research Center of Soil and Water Conservation and Ecological Environment), 2013.
[11] 康绍忠. 土壤-植物-大气连续体水分传输动力学及其应用. 力学与实践, 1993, 15(1): 11-19. KANG S Z. Dynamics of water transport in soil-plant-atmosphere continuum and its application. Mechanics in Engineering, 1993, 15(1): 11-19.
[12] 苗青霞, 方燕, 陈应龙. 小麦根系特征对干旱胁迫的响应. 植物学报, 2019, 54(5): 652-661. doi: 10.11983/CBB19089 MIAO Q X, FANG Y, CHEN Y L. Response of wheat root characteristics to drought stress. Chinese Bulletin of Botany, 2019, 54(5): 652-661. doi: 10.11983/CBB19089
[13] BASU S, RAMEGOWDA V, KUMAR A, PEREIRA A. Plant adaptation to drought stress. F1000Research, 2016, 5: 1554.
[14] MANOJ K, RAJU S, SATOSHI O, YUSAKU U, MICHAEL G S, SATEESH K. Drought response in wheat: Key genes and regulatory mechanisms controlling root system architecture and transpiration efficiency. Frontiers in Chemistry, 2017(5): 106. doi: 10.3389/fchem.2017.00106
[15] KOEVOETS I T, HENKV J, ELZENGA J T M, TESTERINK C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Frontiers in Plant Science, 2016, 7: 1335.
[16] FROMM H. Root plasticity in the pursuit of water. Plants, 2019, 8(7): 236. doi: 10.3390/plants8070236
[17] SERGIO J, JIHENE D, DIEGO G, MORET D, IRIGOYEN J J, MORENO M A, GOGORCENA Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiology, 2013(10): 1061-1075.
[18] 李文娆, 李小利, 张岁岐, 山仑. 水分亏缺下紫花苜蓿和高粱根系水力学导度与水分利用效率的关系. 生态学报, 2011, 31(5): 1323-1333. LI W R, LI X L, ZHANG S Q, SHAN L. The root system hydraulic conductivity and water use efficiency of alfalfa and sorghum under water deficit. Acta Ecologica Sinica, 2011, 31(5): 1323-1333.
[19] 王荣荣, 王海琪, 蒋桂英, 尹豪杰, 谢冰莹, 张婷. 2个不同抗旱性小麦品种耗水特征及根系生理特性对开花期干旱的响应. 水土保持学报, 2022, 36(4): 253-264. doi: 10.13870/j.cnki.stbcxb.2022.04.032 WANG R R, WANG H Q, JIANG G Y, YIN H J, XIE B Y, ZHANG T. Responses of water consumption and root physiological characteristics of two different drought-tolerant wheat varieties to anthesis drought. Journal of Soil and Water Conservation, 2022, 36(4): 253-264. doi: 10.13870/j.cnki.stbcxb.2022.04.032
[20] 忽雪琦, 李东阳, 严加坤, 张岁岐. 干旱胁迫下外源茉莉酸甲酯对玉米幼苗根系吸水的影响. 植物生理学报, 2018, 54(5): 991-998. doi: 10.13592/j.cnki.ppj.2018.0136 HU X Q, LI D Y, YAN J K, ZHANG S Q. Impact of exogenous methyl jasmonate on water absorption of maize roots under drought stress. Plant Physiology Journal, 2018, 54(5): 991-998. doi: 10.13592/j.cnki.ppj.2018.0136
[21] 张海旺, 钟启文, 娄祥云, 杨世鹏, 谭龙, 孙雪梅, 王丽慧. 干旱胁迫对不同基因型菊芋生理特性的影响. 植物生理学报, 2022, 58(12): 2312-2320. doi: 10.13592/j.cnki.ppj.100184 ZHANG H W, ZHONG Q W, LOU X Y, YANG S P, TAN L, SUN X M, WANG L H. Effects of drought stress on physiological characteristics of different genotypes of Helianthus tuberosus. Plant Physiology Journal, 2022, 58(12): 2312-2320. doi: 10.13592/j.cnki.ppj.100184
[22] 于国红, 刘朋程, 郝洪波, 崔海英, 郭安强, 李明哲. 不同基因型谷子对干旱胁迫的调控机制. 植物营养与肥料学报, 2022, 28(1): 157-167. doi: 10.11674/zwyf.2021134 YU G H, LIU P C, HAO H B, CUI H Y, GUO A Q, LI M Z. Regulation mechanism of drought resistance in different genotypes of foxtail millet. Journal of Plant Nutrition and Fertilizers, 2022, 28(1): 157-167. doi: 10.11674/zwyf.2021134
[23] 曾令霜, 李培英, 孙宗玖, 孙晓梵. 两类新疆狗牙根抗旱基因型抗氧化酶保护系统及其基因表达差异分析. 草业学报, 2022, 31(7): 122-132. doi: 10.11686/cyxb2021178 ZENG L S, LI P Y, SUN Z J, SUN X F. Analysis of antioxidant enzyme protection systems and gene expression differences in two Xinjiang bermudagrass genotypes with contrasting drought resistance. Acta Prataculturae Sinica, 2022, 31(7): 122-132. doi: 10.11686/cyxb2021178
[24] 赵小强, 陆晏天, 白明兴, 徐明霞, 彭云玲, 丁永福, 庄泽龙, 陈奋奇, 张大志. 不同株型玉米基因型对干旱胁迫的响应分析. 草业学报, 2020, 29(2): 149-162. ZHAO X Q, LU Y T, BAI X M, XU M X, PENG Y L, DING Y F, ZHUANG Z L, CHEN F Q, ZHANG D Z. Responses of maize genotypes with different plant architecture to drought stress. Acta Prataculturae Sinica, 2020, 29(2): 149-162.
[25] SHUAI J, LI Z, WEI S, WANG Y, MIN Y. Bioactive constituents of Glycyrrhiza uralensis (Licorice): Discovery of the effective components of a traditional herbal medicine. Journal of Natural Products, 2017, 79(2): 281-292.
[26] 盛玉章. 水盐胁迫及施肥对甘草和秦艽品质的影响. 杨凌: 西北农林科技大学硕士学位论文, 2022. SHENG Y Z. Effects of water and salt stress and fertilization on the quality of Glycyrrhiza uralensis Fisch. and G. macrophylla Pall. Master Thesis. Yangling: Northwest Agriculture and Forestry University, 2022.
[27] 耿广琴, 谢晓蓉. 旱盐双重胁迫对乌拉尔甘草幼苗生理生化特性的影响. 草业科学, 2018, 35(9): 2166-2173. doi: 10.11829/j.issn.1001-0629.2017-0464 GENG G Q, XIE X R. Effect of drought and salt stress on the physiological and biochemical characteristics of Glycyrrhiza uralensis. Pratacultural Science, 2018, 35(9): 2166-2173. doi: 10.11829/j.issn.1001-0629.2017-0464
[28] 李宗谕, 刘福顺, 刘秀岩, 杨世海. 盐碱胁迫和干旱胁迫对胀果甘草种子萌发及幼苗生长和内源激素影响. 时珍国医国药, 2020, 31(6): 1464-1467. doi: 10.3969/j.issn.1008-0805.2020.06.062 LI Z Y, LIU F S, LIU X Y, YANG S H. Effects of saline-alkali stress and drought stress on seed germination, seedling growth and endogenous hormones of Glycyrrhiza inflata Batalin. Lishizhen Medicine and Materia Medica Research, 2020, 31(6): 1464-1467. doi: 10.3969/j.issn.1008-0805.2020.06.062
[29] 张东, 刘艳, 张晗, 王洋, 张子健, 刘美岑. 甘草叶片渗透调节物质及蔗糖代谢相关酶对干旱胁迫的响应特性. 西北植物学报, 2020, 40(5): 819-827. ZHANG D, LIU Y, ZHANG H, WANG Y, ZHANG Z J, LIU M C. Response of osmotic regulator and sucrose metabolization-related enzymes to drought stress in Glycyrrhiza uralensis. Acta Botanica Boreali-Occidentalia Sinica, 2020, 40(5): 819-827.
[30] 安钰, 张清云, 李生兵, 刘华. 干旱胁迫及复水对甘草叶片抗氧化酶活性和光合特性的影响. 宁夏农林科技, 2021, 62(7): 1-5. doi: 10.3969/j.issn.1002-204x.2021.07.001 AN Y, ZHANG Q Y, LI S B, LIU H. Effect of drought stress and rehydration on antioxidant enzyme activity and photosynthetic characteristic of Glycyrrhiza uralensis. Journal of Ningxia Agriculture and Forestry Science and Technology, 2021, 62(7): 1-5. doi: 10.3969/j.issn.1002-204x.2021.07.001
[31] 关思静, 王楠, 徐蓉蓉, 葛甜甜, 高静, 彭亮, 张岗, 陈莹. 甘草幼苗响应干旱胁迫的光合、抗氧化特性及转录组分析. 草业科学, 2021, 38(11): 2176-2190. GUAN S J, WANG N, XU R R, GE T T, GAO J, PENG L, ZHANG G, CHEN Y. Photosynthetic, antioxidant enzyme activity, and transcriptome sequencing analyses of Glycyrrhiza uralensis seedings in response to drought stress. Pratacultural Science, 2021, 38(11): 2176-2190.
[32] 刘长乐, 寇佩雯, 许祎珂, 张永生, 高静, 贺壮壮, 李帅帅, 黄文静. 逆境胁迫和植物生长调节剂对甘草种子萌发的影响. 中国野生植物资源, 2022, 41(2): 14-20. LIU C L, KOU P W, XU Y K, ZHANG Y S, GAO J, HE Z Z, LI S S, HUANG W J. Effect of adversity stress and plant growth Regulator on seed germination of Glycyrrhiza uralensis Fish. Chinese Wild Plant Resources, 2022, 41(2): 14-20.
[33] YAN J K, ZHANG N N, WANG N, LI Y P, ZHANG S Q, WANG S W. Variations in adaptation strategies of wheat cultivar replacements under short-term osmotic stress. Pakistan Journal of Botany, 2016, 48(3): 917-924.
[34] ZHAO C X, DENG X P, SHAN L, STEUDLE E, ZHANG S Q, YE Q. Changes in root hydraulic conductivity during wheat evolution. Journal of Integrative Plant Biology, 2005, 47(3): 302-310. doi: 10.1111/j.1744-7909.2005.00043.x
[35] 朱铁霞, 高阳, 高凯, 李志华. 干旱胁迫下菊芋各器官生物量及物质分配规律. 生态学报, 2019, 39(21): 8021-8026. ZHU T X, GAO Y, GAO K, LI Z H. Organ biomass and resource allocation in response to drought stress in jerusalem artichoke. Acta Ecologica Sinica, 2019, 39(21): 8021-8026.
[36] YIN C Y, BERNINGER F, LI C Y. Photosynthetic responses of Populus przewalskii subjected to drought stress. Photosynthetica, 2006, 44(1): 62-68.
[37] 王传旗. 水分亏缺及复水下老芒麦生理生态特性及耐旱性研究. 西宁: 青海大学博士学位论文, 2022. WANG C Q. Studies on physiological ecology characteristics and drought tolerance of Elymus sibiricus under water deficit and rewatering. PhD Thesis. Xining: Qinghai University, 2022.
[38] 项雯慧, 张林平, 王洋, 刘艳. 不同浓度PEG胁迫对甘草幼苗光合特性的影响. 内蒙古农业大学学报(自然科学版), 2020, 41(1): 19-24. doi: 10.16853/j.cnki.1009-3575.2020.01.004 XIANG H W, ZHANG L P, WANG Y, LIU Y. Effects of different concentrations of PEG-6000 stress on photosynthetic characteristics of Glycyrrhiza young seedlings. Journal of Inner Mongolia Agricultural University (Natural Science Edition), 2020, 41(1): 19-24. doi: 10.16853/j.cnki.1009-3575.2020.01.004
[39] UTKHAO W, YINGJAJAWAL S. Changes in leaf gas exchange and biomass of Eucalyptus camaldulesis in response to increasing drought stress induced by polyethylene glycol. Trees, 2015, 29(5): 1581-1592. doi: 10.1007/s00468-015-1240-1
[40] 刘歌畅, 岳丽华, 刘春海, 赵鹏智, 邓子易, 李玉灵. 干旱胁迫对俄罗斯大果沙棘幼苗光合荧光参数的影响. 北方园艺, 2022, 517(22): 16-23. LIU G C, YUE L H, LIU C H, ZHAO P Z, DENG Z Y, LI Y L. Effects of drought stress on photosynthetic fluorescence parameters of Hippophae rhamnoides Linn. Seedings. Northern Horticulture, 2022, 517(22): 16-23.
[41] ZHAO J, LANG Y, ZHANG S Y, ZHAO Q K, ZHANG C Y, XIA J B. Photosynthetic characteristics and chlorophyⅡ a fluorescence transient in Lonicera japonica under drought stress. Acta Physiologiae Plantarum, 2019, 41(7): 124.
[42] 王根轩, 廖建雄, 吴冬秀. 荒漠条件下甘草气孔振荡的水被动证据. 植物学报, 2001, 43(1): 41-45. WANG G X, LIAO J X, WU D X. Evidence of hydropassive movement in stomatal oscillations of Glycyrrhiza inflata under desert conditions. Journal of Integrative Plant Biology, 2001, 43(1): 41-45.
[43] 刘文瑜, 杨发荣, 黄杰, 魏玉明, 李健荣. 干旱胁迫对藜麦幼苗生长和叶绿素荧光特性的影响. 干旱地区农业研究, 2019, 37(4): 171-177. LIU W Y, YANG F R, HUANG J, WEI Y M, LI J R. Effect of drought stress on chlorophyⅡ fluorescence properties and seedling growth of quinoa seedlings. Agriculture Research in the Arid Areas, 2019, 37(4): 171-177.
[44] GAO H, BAI N, ZANG Y, ZHANG Y J, WANG L, WANG E J, TIAN Y Y, GUO Y Y, YAN F, LI Y H, ZHANG H. Drought stress alters gas exchange, chlorophyⅡ fluorescence, and antioxidant enzyme activities in Glycyrrhiza uralensis in the Hexi Corridor, China. Russian Journal of Plant Physiology, 2022, 69(6): 123.
[45] GUO Y Y, YU H Y, KONG D S, FAN F, ZHANG Y J. Effects of drought stress on growth and chlorophyⅡ fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica, 2016, 54(4): 524-531. doi: 10.1007/s11099-016-0206-x
[46] WANG J, LU W, TONG Y, YANG Q. Leaf morphology, photosynthetic performance, chlorophyⅡ fluorescence, stomatal development of lettuce ( Lactucasativa L.) exposed to different ratios of red light to blue light. Frontiers in Plant Science, 2016, 7: 250.
[47] 庞进平, 王永生. 油菜幼苗光合及叶绿素荧光参数对干旱胁迫的响应及其抗旱性分析. 西北植物学报, 2023, 43(2): 276-284. doi: 10.7606/j.issn.1000-4025.2023.02.0276 PANG J P, WANG Y S. Photosynthetic and chlorophyⅡ fluorescence responses of rape seedlings to drought stress and its drought resistance evaluation. Acta Botanica Boreali-Occidentalia Sinica, 2023, 43(2): 276-284. doi: 10.7606/j.issn.1000-4025.2023.02.0276
[48] YAN M, ZHANG C, LI H, ZHANG L, REN Y, CHEN Y, CAI H, ZHANG S. Root pruning improves maize water-use efficiency by root water absorption. Frontiers in Plant Science, 2023, 13: 1023088. doi: 10.3389/fpls.2022.1023088
[49] ZHANG W J, XIE Z C, ZHANG X J, LANG D Y, ZHANG X H. Growth-promoting bacteria alleviates drought stress of G. uralensis through improving photosynthesis characteristics and water status. Journal of Plant Interactions, 2019, 14(1): 580-589. doi: 10.1080/17429145.2019.1680752
[50] 蒋涛, 郑文革, 余新晓, 贾国栋, 孜尔蝶·巴合提, 张欢, 王渝淞, 王玥璞, 丁兵兵. 北京山区干旱胁迫下侧柏叶片水分吸收策略. 生态学报, 2022, 42(4): 1429-1440. JIANG T, ZHENG W G, YU X X, JIA G D, Zierdie·Baheti, ZHANG H, WANG Y S, WANG Y S, WANG Y P, DING B B. Leaf water absorption of Platycladus orientalis under drought stress in Beijing. Acta Ecologica Sinica, 2022, 42(4): 1429-1440.
[51] 王常贵, 管绍淳, 杨戈. 新疆甘草属植物的解剖学研究. 干旱区研究, 1984(2): 44-48. doi: 10.13866/j.azr.1984.02.009 WANG C G, GUAN S C, YANG G. Anatomical study on Glycyrrhiza L. plants in Xinjiang. Arid Zone Research, 1984(2): 44-48. doi: 10.13866/j.azr.1984.02.009
[52] 李文娆, 张岁岐, 丁圣彦, 山仑. 干旱胁迫下紫花苜蓿根系形态变化及与水分利用的关系. 生态学报, 2010(19): 5140-5150. LI W R, ZHANG S Q, DING S Y, SHAN L. Root morphological variation and water use in alfalfa under drought stress. Acta Ecologica Sinica, 2010(19): 5140-5150.
[53] HILL D, NELSON D, HAMMOND J, BELL L. Morphophysiology of potato ( Solanum tuberosum) in response to drought stress: Paving the way forward. Frontiers in Plant Science, 2021, 11: 597554. doi: 10.3389/fpls.2020.597554
[54] 苗果园, 高志强, 张云亭, 尹钧, 张爱良. 水肥对小麦根系整体影响及其与地上部相关的研究. 作物学报, 2002, 28(4): 445-450. MIAO G Y, GAO Z Q, ZHANG Y T, YIN J, ZHANG A L. Effect of water and fertilizer to root system and its correlation with tops in wheat. Acta Agronomica Sinica, 2002, 28(4): 445-450.
[55] XIA J B, ZHANG S Y, LI T, LIU R H, GUANG C. Effect of continuous cropping generations on each component biomass of poplar seedlings during different growth periods. The Scientific World Journal, 2014(2): 618421.
[56] 陈明涛, 赵忠. 干旱对4种苗木根系特征及各部分物质分配的影响. 北京林业大学学报, 2011, 33(1): 16-22. doi: 10.13332/j.1000-1522.2011.01.016 CHEN M T, ZHAO Z. Effects of drought on root characteristics and mass allocation in each part of seedlings of four tree species. Journal of Beijing Forestry University, 2011, 33(1): 16-22. doi: 10.13332/j.1000-1522.2011.01.016
[57] 张岁岐. 根冠关系对作物水分利用的调控. 杨凌: 西北农林科技大学博士学位论文, 2001. ZHANG S Q. Regulation of root-shoot relationship on crop water use. PhD Thesis. Yangling: Northwest Agriculture and Forestry University, 2001.
[58] 马富举, 李丹丹, 蔡剑, 姜东, 曹卫星, 戴廷波. 干旱胁迫对小麦幼苗根系生长和叶片光合作用的影响. 应用生态学报, 2012, 23(3): 724-730. doi: 10.13287/j.1001-9332.2012.0097 MA F J, LI D D, CAI J, JIANG D, CAO W X, DAI T B. Responses of wheat seedings root growth and leaf photosynthesis to drought stress. Chinese Journal of Applied Ecology, 2012, 23(3): 724-730. doi: 10.13287/j.1001-9332.2012.0097
[59] YAN M, ZHANG L, REN Y, ZHANG T, ZHANG S, LI H, CHEN Y, ZHANG S. The higher water absorption capacity of small root system improved the yield and water use efficiency of Maize. Plants, 2022, 11(17): 112300.
-
期刊类型引用(3)
1. 戈艳,蔺万煌,高鸿永. 中药材甘草植物抗旱性及其在沙漠治理中研究进展. 农业灾害研究. 2024(09): 49-51 .  百度学术
百度学术
2. 金照鑫. 脱硫石膏配施沼液肥对重度盐碱土电导率及油菜生长的影响. 甘肃科技. 2024(10): 110-114 .  百度学术
百度学术
3. 徐芬芬,周油涌,徐兵. 萘乙酸(NAA)对模拟干旱胁迫下上饶白玉豆种子萌发及其幼苗根系生长的影响. 上饶师范学院学报. 2024(06): 50-56 .  百度学术
百度学术
其他类型引用(5)



 下载:
下载: