第一作者:谌馥佳(1985-),女,湖北黄陂人,讲师,博士,研究方向为生物制药。E-mail:[email protected]
MicroRNA(miRNA)作为一种内源性的非编码RNA,以影响靶基因表达的方式来调节机体功能。miRNA能调控哺乳动物性腺(睾丸和卵巢)的发育,促进精子与卵母细胞的分化成熟,影响受精卵的发育过程,并可能作为诊断生殖疾病卵巢癌的重要指标。本文对miRNA的生物合成过程,miRNA在哺乳动物性腺发育以及卵巢癌变过程中的调控功能进行了综述,提出该领域主要发展方向是miRNA在哺乳动物性腺发育中的体内功能研究,希望以分子生物学和生物信息学手段,从分子、系统等不同角度阐明单一某种miRNA在哺乳动物性腺发育及卵巢癌变过程中的具体调节机制和信号通路情况。这为研究哺乳动物生殖疾病,选育畜牧生产中优质品种提供了新的思路和途径。
MicroRNA (miRNA), a class of endogenous noncoding RNAs, plays a role in regulation of the body’s physiological function by affecting target gene expression. miRNAs can regulate the development of gonads (testicles and ovaries), promote maturation and differentiation of sperm and oocytes, and affect the growth of oosperm. miRNAs can be used as an important index in diagnosis of ovarian cancer. This paper summarizes miRNAs’ biogenesis and its regulation in mammal gonadal development and the progress of ovarian cancer; research has mainly focused on these functions in vivo. The regulation mechanisms and signalling pathways of miRNAs in gonadal development of mammals and ovarian cancer is revealed through molecules, systems, and other aspects of biology through molecular biology and bioinformatics. We provide new thoughts and approaches for reproductive disease research and high-quality animal breeding.
MicroRNA(miRNA)作为近年来新兴的研究热点, 是一类内源性的非编码RNA, 长21~24个核苷酸, 通常与靶mRNA 3'端的非翻译区结合, 从而诱导靶mRNA的降解或抑制靶mRNA的表达, 它们广泛存在于植物、微生物、线虫、动物和人类的细胞中[1]。1993年人们首次在秀丽新小杆线虫(Caenorhabditis elegans)中发现了控制线虫时序性发育的小分子miRNA:lin4基因[2]。接着在线虫中发现了let7基因, 该miRNA具有转录后调节功能[3]。之后许多miRNA被相继发现[4]。在人类基因组中, 2/3左右的人类基因由miRNA调控, 在人体内形成错综复杂的调控网络, 这些靶基因与miRNA存在着“ 一对多” 或者“ 多对一” 的关系[5]。目前发现miRNA可以有效调控生物体的生长、发育和凋亡等过程, 多种生物的miRNA已由miRBase收录[6]。同生物体其他器官和组织一样, 哺乳动物的性腺及其发育也受miRNA的调控。miRNA能够在哺乳动物的性腺及其组织中广泛表达, 且表达量在性腺的不同发育时期呈现不同变化趋势, 在性腺激素的参与下共同影响性腺发育, 主要是调控睾丸、卵巢的正常发育, 精子与卵母细胞进一步的分化成熟, 以及受精卵的整个发育过程。
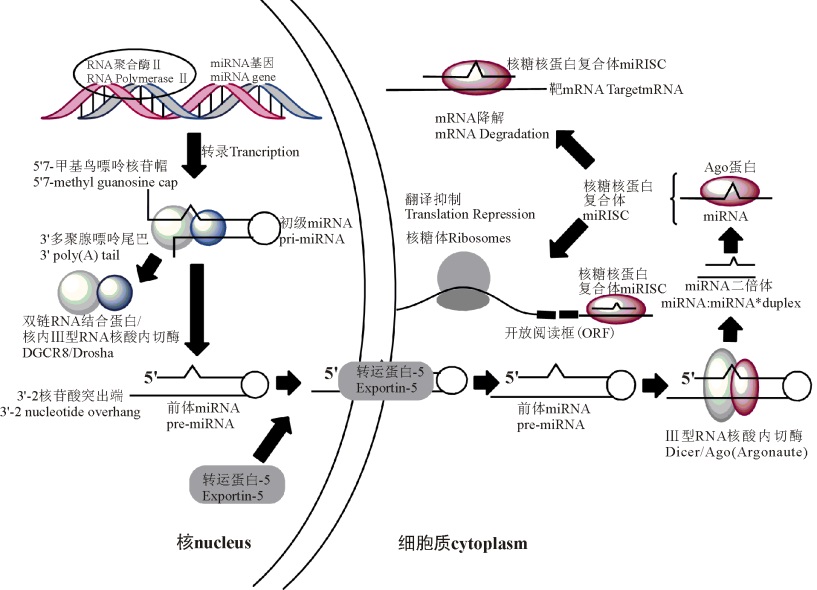
miRNA的生物合成途径通常包括3个步骤[7, 8, 9, 10, 11, 12, 13, 14](图1):1)在RNA聚合酶Ⅱ 的催化作用下将miRNA转录成初级miRNA(pri-miRNA)。2)pri-miRNA被按步骤分区处理之后, 最终以成熟的miRNA形式存在于细胞中; 此过程中, 首先pri-miRNA在核内Ⅲ 型RNA核酸内切酶Drosha作用下, 被剪切成前体miRNA(pre-miRNA), 该前体miRNA 70~90 bp, 接着Drosha酶能够与DGCR8蛋白(即双链RNA结合蛋白)结合而成微型络合物[7]; 然后在Expotin-5输出蛋白的作用下, 由辅助因子Ran-GTP参与, pre-miRNA从核内转运至胞质, 在胞质内Ⅲ 型RNA核酸内切酶Dicer剪切作用下, 形成一段双链miRNA(约22 bp)[8]; 最后双链miRNA解螺旋后形成成熟的单链miRNA[9]。3)成熟miRNA能够与miRISC结合发挥功效。miRISC是一种miRNP即核糖核蛋白复合体(图1), 也是RNA诱导相关的沉默复合体。在核糖核蛋白复合体上, miRNA一般以两种不同的方式来调控靶基因的表达, miRNA 和Argonaute家族靶基因结合蛋白的互补程度决定了抑制翻译或降解靶mRNA的方式:部分互补抑制靶mRNA的翻译, 完全互补则会使其降解[10, 11], 具体机制尚不清楚。miRNA或被羁留在核糖体上[12], 或被补充到Pbodies中, Pbodies为细胞质处理小体, 最后该miRNA被降解[13]。
图1 哺乳动物miRNA生物合成途径(Fig. 1 The biosynthetic pathway of miRNA of mammals)
睾丸miRNA表达情况存在着物种差异性和阶段特异性。Yan等[15]以恒河猴(Macaca mulatta)、人(Homo sapiens)等灵长类动物的睾丸组织为材料, 研究miRNA的表达情况, 结果发现, 共有66个(占总数的26.4%)miRNA在恒河猴睾丸中差异性表达, 如hsa-miR-493-3p、hsa-miR-376b、hsa-miR-222等, 其中有23个miRNA表达上调, 其余的miRNA下调; 而在人睾丸组织中检测到76个(占总数的31.3%)miRNA差异表达, 如hsa-miR-181c、hsa-let-7e、hsa-miRNA-219等, 其中有28个miRNA表达上调, 48个miRNA表达下调; 另外miRNA的表达在灵长类睾丸的不同发育阶段存在着阶段特异性差异, 例如hsa-miR-154、hsa-miR-181c、hsa-miR-181d和hsa-miR-487b等在未成熟的睾丸中表达上调, 在成熟的睾丸组织中表达下调。这些miRNAs可能通过调控精母细胞、精细胞的形成及分化过程来调控整个精子的发生过程。在研究不同miRNAs在猪睾丸发育过程中的作用时发现, miR-122在睾丸发育成熟的过程中表达量升高, 而miR-221却表达量下调, 这说明miR-122与miR-221功能不同[16]。接着建立了关于猪睾丸的小RNA文库, 结果发现在猪睾丸组织中共有122个miRNAs可以进行差异性表达[17], 目前已经逐步开展其功能验证工作。采用生物信息学与实时荧光定量PCR(Real-time PCR)等手段探讨绵羊(Ovis aries)妊娠胎儿中睾丸miRNA在各妊娠时期的表达特点时发现, 30种miRNA的表达呈现出显著性差异, 有13种miRNA(如miRNA-99b、miRNA-149等)在妊娠初期(即第42天)的表达量显著升高, 另外17种miRNA(如miRNA-19b、miRNA-204等)在妊娠中期(即第75天)的表达量显著升高[18]。在细胞内外因素的调控下, 睾丸miRNA的表达受到影响, 睾丸基因表达乃至睾丸的发育和精子形成过程也受到miRNA的调控。这些细胞内外因素包括激素、转录因子、酶和蛋白等。例如人们发现小鼠(Mus musculus)睾丸细胞miRNA的表达是由雄激素、酶或蛋白质共同调节的[19]。另有研究表明, 雄性生殖腺如果缺乏第2类的核糖核酸酶Ⅲ , 可能会对成熟miRNA的形成过程产生阻碍作用, 最终导致雄性不育[20]。
成熟精子是一种单倍体生殖细胞, 其特殊之处在于其生成过程需要相关因子一系列的精准调控。例如在小鼠精子的精原细胞(prospermatogonia)中miR-17-92、miR-290-295家族能够高效表达[21]。敲除了miR-17-92后, 小鼠的睾丸会缩小, 精子产生数量和质量都会下降[22], 表明miR-17-92与miR-106b-25协作调控精子发育与成熟过程。小鼠精子细胞中的miR-34c能够高度表达, 与靶基因ATF1(活化转录因子)互作引起精子细胞等雄性生殖细胞凋亡[23]。现阶段, 关于miRNA在整个精子成熟过程中调控作用的研究主要集中在前期发育阶段, 即是由原始生殖细胞(primordial germ cells, PGCs)至精原细胞的形成过程, 而对从初级精母细胞(spermatocyte)至成熟精子细胞(spermatozoa)的后期发育阶段研究较少[21, 22, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38](表1)。
表1 牧草及粮食作物调查有效样本数量(Table 1 Effective sample size of forage and grain crops)
| 作用部位Acting site | miRNA | 参考文献Reference |
|---|---|---|
| 调控原始生殖细胞(PGCs)增殖和发育 Modulate the proliferation and development of PGCs | miR-323, miR-141, miR-200(a, c) | [21] |
| miR-9 | [21, 25] | |
| miR-19(a, b) | [25] | |
| miR-let-7(a, d, e, f, g) | [21, 26-27] | |
| miR-17-92, miR-106b-25 | [22] | |
| miR-302-367 | [28] | |
| miR-290-295 | [29] | |
| 调控精原干细胞(Sperma-togonial stem cells, SSCs) 更新和分化 Modulate the update and differentiation of SSCs | miR-let-7(7, a, d, c, d, e) | [30] |
| miR-21 | [31] | |
| miR-34c | [32] | |
| miR-148 | [27] | |
| miR-20, miR-106a | [33] | |
| 调控精原细胞(Spermato-gonia)分化 Modulate the differentiation of Spermatogonia | iR-221, miR-222 | [34] |
| miR-17-92, miR-106b-25 | [26] | |
| miR-449 | [35] | |
| 调控减数分裂过程Modulate the meiotic stage | miR-24, miR-214 | [36] |
| 调控精子细胞的形成Modulate spermatogenesis | miR-22a, miR-34c | [37] |
| 维持成熟的精子结构Maintain the structure of mature sperm | miR-888 | [38] |
卵泡发育、排卵等生理过程, 由大量基因表达的产物以及miRNAs参与调控, 以确保这些基因在表达和信息交流过程中的时空准确性。Tang等[39]在小鼠成熟卵细胞中发现miR-16、let-7等家族的多种miRNAs。后来又从女性卵母细胞颗粒和卵丘细胞中发现miR-17-5p、miR-23a、miR-23b等多种miRNAs[40]。在牛卵巢miRNA的研究中发现, miRNA克隆共74个, 其中新克隆有24个, Bta-miR-29a能够在卵泡细胞的整个发育过程中特异性表达[41]。在小鼠新生儿(NB)的卵巢中, miRNA-let-7家族和mmu-mir-322、mmu-mir-672家族等的表达最为丰富, 后两者是一类与X染色体连锁的miRNA, 其中mmu-mir-672家族通常会在性腺中优先表达[42]。
有研究发现, miRNA在小鼠卵母细胞发育过程中发挥着重要功能, 能够敲除内切核糖核酸酶Dicer, 从而阻止前体miRNA生成miRNA, 致使原始生殖细胞发育过程受阻, 减数分裂停滞[43, 44]。研究发现, 在减数分裂时期卵母细胞中共有861个转录产物即mRNA表达上调, 173个mRNA表达下调, 这说明, miRNA调节卵母细胞成熟的机制是通过调控这些mRNA的表达来实现的[45]。miRNA-224通过作用于SMAD4靶基因来调控由转化生长因子β (TGF-β )介导的小鼠卵母颗粒细胞的增殖途径和激素分泌过程[46]。miR-21的功能是阻碍小鼠排卵前的卵母颗粒细胞凋亡[47]。
性腺激素在正常生殖过程、生殖紊乱疾病以及与雌激素相关的癌症中起着重要作用[48]。以转染的原代人卵母颗粒细胞为材料, 检测miRNAs对性腺激素的影响, 发现36种miRNAs抑制孕酮的产生, 16种促进孕酮的产生, 58种抑制睾酮的产生, miR-107促进睾酮的产生, 51种抑制雌激素的生成[49]。这说明miRNAs会影响卵巢类固醇激素的生成。
然而, 类固醇激素也影响miRNA 的生成。Pan等[50]用乙酸甲羟孕酮和17β -雌二醇处理原代培养的腺上皮细胞和人子宫内膜间质, 结果发现, miR-20a、miR-21和miR-26a这3种miRNA的表达受到影响。活化的雌激素受体(estrogen receptor, ER)与核内Ⅲ 型RNA核酸内切酶(Drosha)互作可以抑制pri-miRNAs的成熟, 从而增大这些miRNAs的靶基因表达量[51]。在人体处于增殖期和分泌期时, miRNAs在子宫内膜中的表达有明显的差异, 研究发现有12个miRNAs在分泌期时表达量显著升高, 这是由于孕酮在该时期起到了关键性作用[52]。
另外, 促性腺激素也能调节miRNAs的生成。黄体生成素(LH)对人排卵期卵泡颗粒细胞中的13个miRNAs有调控作用[53]。卵泡刺激素(FSH)可以抑制小鼠卵母颗粒细胞中let-7a、miR-143与miR-125b这3个miRNAs的表达[54]。人绒毛膜促性腺激素(HCG)能够下调小鼠卵母颗粒细胞和卵泡细胞中的个别miRNA的表达量[55]。
上皮性卵巢癌(EOC)是最严重的女性恶性肿瘤之一[56], 每年死于该疾病的女性约有12万人之多, 现阶段虽然在EOC的诊断与治疗领域取得了一定的进展, 但是对其发育过程分子机制的研究较少, 目前研究表明miRNA能够调控卵巢癌的发生过程[57]。卵巢癌中miRNA的表达通常用基因芯片表达谱来检测, 卵巢癌中的部分miRNAs其表达量明显上调, 如miR-141、miR-200a、miR-200b和miR-200c, 而另外一些miRNAs(如miR-199a、miR-125b1、miR-145和miR-140)表达量则显著下调, 可以根据卵巢癌的组织病理特点对这些发生变化的miRNAs进行分类[58]。某些miRNAs的表达量上调可能是由于卵巢癌细胞中DNA的甲基化, 例如miR-21、miR-205等。目前miRNA在许多肿瘤的发生发展过程中属于抑癌基因, 但是其分子机制尚不清楚[59]。原代肿瘤细胞中的miRNA表达量与基因组以及表观的变化有一定的相关性[60], 一些具有抑癌基因功能的miRNA会在卵巢癌的晚期表达, 且表达量下调。
另有研究表明, 某些miRNAs可能调节肿瘤的形成, 如miR-214和miR-200a, miR-214是在卵巢癌中表达上调最显著的miRNA, 能够抑制PTEN抑癌基因的表达, 从而激活Akt信号通路; 因为缺少3'非翻译区的PTEN抑癌基因表达载体, Akt的抑制剂或者过表达可能会对miR-214诱导的细胞存活具有抑制效果[61]。这说明卵巢癌中某些miRNA表达量的变化, 有可能是癌变信号, 这些miRNA可能成为潜在诊断及治疗卵巢癌的重要指标。
哺乳动物的个体发育过程由受精卵开始, miRNA在精卵结合后的受精卵发育成熟过程中, 也发挥了重要的调节功能。受精卵中的母源miRNAs含量最多的成员有let-7家族和miR-17-92家族[39], 其miRNA的表达谱在早期胚胎个体发育的各个阶段呈现一定的差异, 其表达量在卵子成熟至受精卵的形成, 再到早期胚胎的发育过程中呈现先下降后上调的现象。小鼠早期胚胎发育过程受到由miR-196a调控NOBOX靶基因(新生儿卵巢同源盒基因)表达的影响, miR-196a表达量持续上升, NOBOX基因表达量下降[62]。miR-135a通过下调靶基因SIAH1A的表达实现对早期胚胎体外发育的调控[63]。miR-181a 通过抑制NPM2(核质, Nucleoplasm 2)基因的表达调控卵母细胞的激活与早期胚胎的发育过程, 在此过程中, miR-181a与NPM2基因的表达一直呈现负相关性[64]。某些母源性miRNA对早期胚胎的发育至关重要, 将胞质内Ⅲ 型RNA核酸内切酶(Dicer)突变体小鼠与正常小鼠的基因表达谱进行比较分析发现, miRNA以直接或间接作用对大部分母源性基因的表达进行调控[43]。
miRNA在哺乳动物性腺发育的各个阶段均发挥着重要的功能, 对一系列重要生殖活动(例如精子发生和卵泡发育过程、激素分泌和受精卵发育过程)、生殖疾病及卵巢癌的发生进行调控。miRNA通过与靶mRNA以碱基配对方式进行特异性结合, 降解靶mRNA或者抑制靶mRNA的翻译过程, 从而调控基因表达。研究miRNA对性腺的正常发育及生殖活动的调控功能具有重要的实践意义, 可为研究人类及其他哺乳动物生殖系统疾病提供理论基础, 可为畜牧生产中优质品种的选育提供新的思路和途径。
鉴于性腺发育、生殖以及生殖疾病发生机制的复杂性, 人们对于miRNA的研究尚处于初级阶段, 还存在着一些问题:1)试验材料的广泛性, 对哺乳动物miRNA的研究不但要重点突出, 如小鼠和人, 而且要涉及广泛, 对其他哺乳动物的研究还有待深入; 2)最好采用体外试验与体内试验相结合的研究方法, 目前的研究结果多是体外试验结果, 而对体内功能研究较少; 3)对于单一某种miRNA在哺乳动物生命活动中的作用及其机理也有待更深入的研究, 包括具体的调节网络和信号通路的情况等。
与哺乳动物性腺发育相关的miRNA作为目前研究的热点, 通过大规模测序已经有了初步的发展, 但是仍有大部分miRNA靶基因尚未确定。得益于分子生物学和生物信息学技术的进步[65], 如差异表达谱分析技术、miRISC免疫共沉淀技术、生物信息学等, 开发具备高灵敏度、高通量特异性的miRNA靶基因筛选鉴定方法是目前亟待解决的课题, 以更先进的手段, 重点研究miRNA在哺乳动物性腺发育中的体内功能, 从分子、系统等不同角度阐明单一某种miRNA在哺乳动物性腺发育及卵巢癌变过程中的具体调节机制和信号通路情况, 势必成为该领域主要发展方向。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|


